Chapter 9 - Myoglobin, Hemoglobin
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
What is Hemoglobin? What is Mygolobin? How does their structure and function differentiate them? What structural features do they have in common?
Myoglobin → Tertiary polypeptide single chain
O2 Storage (muscles, etc.)
Hemoglobin → Quarternary Tetramaer (a2,B2)
Two alpha subunits, two beta subunits
O2 transport (in RBC’s)
BOTH are globulin fold (domain) structured
BOTH are ONLY made from alpha-helices (8 alpha helices/subunit)
So since myoglobin is just a single protein unit, it is made of a total of 8 alpha helices
Hemoglobin is made of 4 subunits, so it has 32 alpha helices total
What are the alpha helices surrounding in each subunit? What are stablizing them? Which one is directly bonded to heme, which one is not?
How many heme groups for each?
Alpha helices surround a HEME group.
Proximal His (F8) → anchoring Fe2+
directly bound to heme
Distal His (E7) → Stabilizes O2 binding and reduces CO binding
Not directly bound, hovering above close by
Myoglobin → one Heme group
Hemoglobin → 4 heme groups
Why does the Distal His need to reduce CO binding?
CO has a MUCH HIGHER affinity to bind to heme than does O2. The distal His introduces interferences to prevent CO binding
What is the Fe2+ in the middle bound to? How many binding sites can Fe2+ have?
Bound to four Nitrogen aroms. Can bind to a total of 6 things.
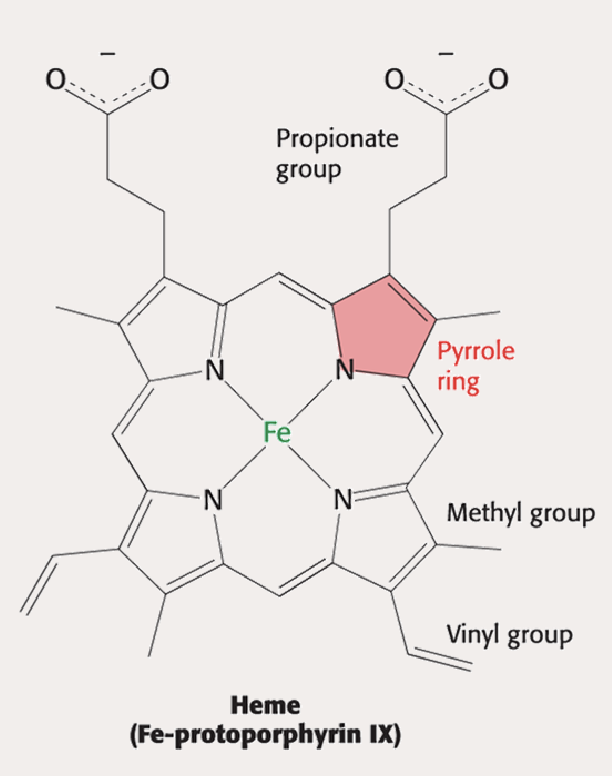
What is the lower part of Fe bound to? What about the upper part? Draw out how the binding looks 3D
Lower site → Bound w/ Proximal Histidine (F8)
Upper site → Left Open for O2 binding, with Distal Histidine hovering above to stabilize the binding and prevent CO
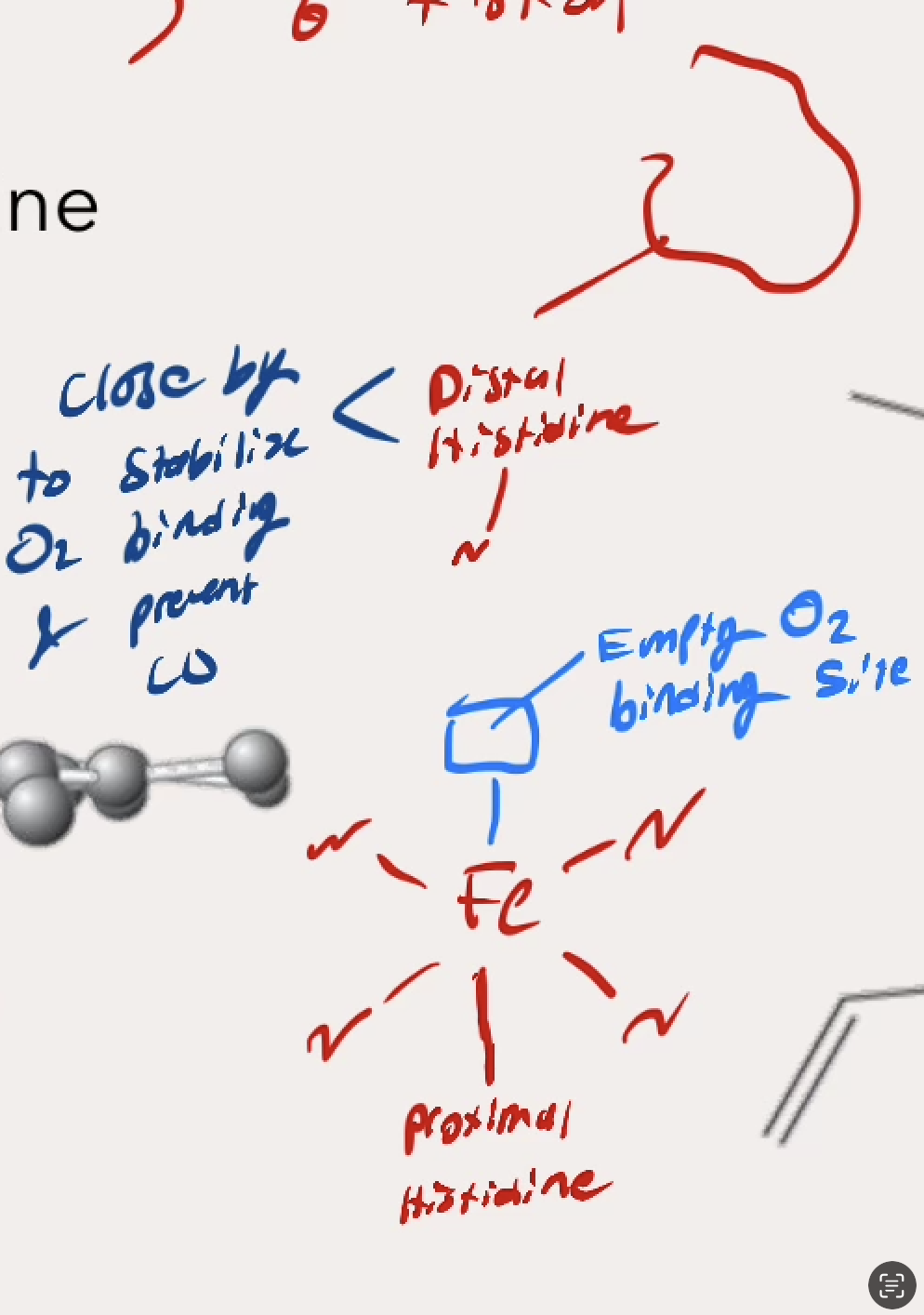
What happens bewtwen the T state and R state of Hemoglobin? In which state is it delivering (relesing) oxygen and in which is it binding to oxygen
T state → The Fe2+ in the middle has low affinity, is not flatly planar
Hole in the middle is wide
DELIVERING (releasing oxygen)
R state → The Fe2+ moves into the flat planar, the Prozimal Histidine shifts, and the Protein twists itself slowly 15 degrees rearrganing for higher affinity.
Hole in the middle has tightened, but still allows for O2 binding
BINDING to oxygen
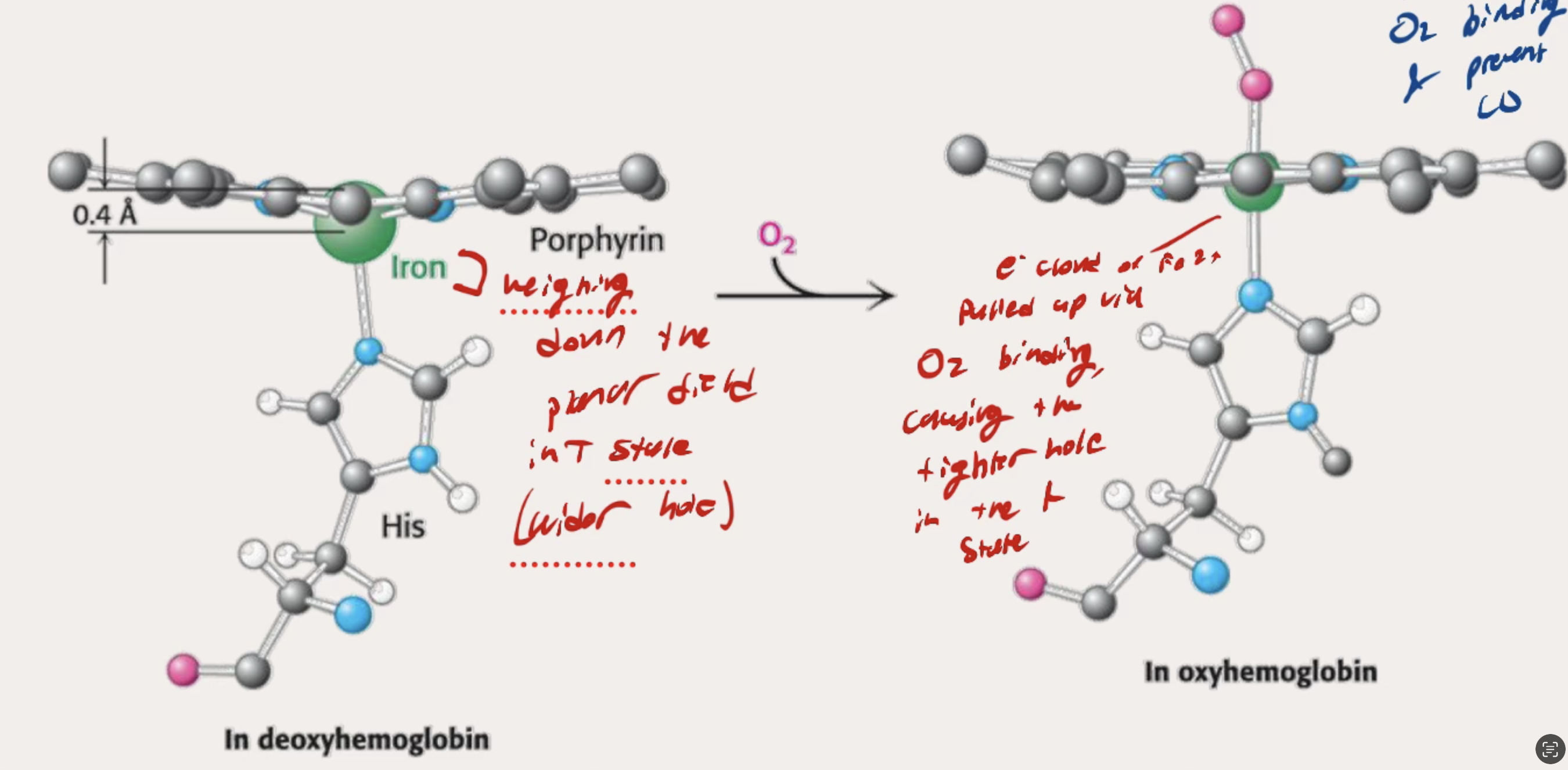
What does it look like molecularly when T state foes to R state with teh presence of O2? What happens?
The iron weighing down the planar field in the T state causes teh wider hole in the protein.
O2 binding pulls up the Iron which causes change throughout protein to have a tighter hole in the R state
What type of MM plot does Myoglboin have? how does it relate to function?
Myoglobin → Hyperbolic Shape (Low P50 (Km) → High affinity
Makes sense beause it’s job is to reaain O2 for storage
Will release O2 at very severe conditions of low pO2 (like hypoxia)
What type of MM plot does Hemoglobin have? how does it relate to function
Hemoglobin → Sigmoidal shape (mixed P50)
T state Hb → HIGH P50 (low affinity for oxygen)
R state Hb → LOW P50 (high affinity for oxygen)
Binds to O2 well enough, but it is better able to release O2 (makes sense because it just transports oxygen)
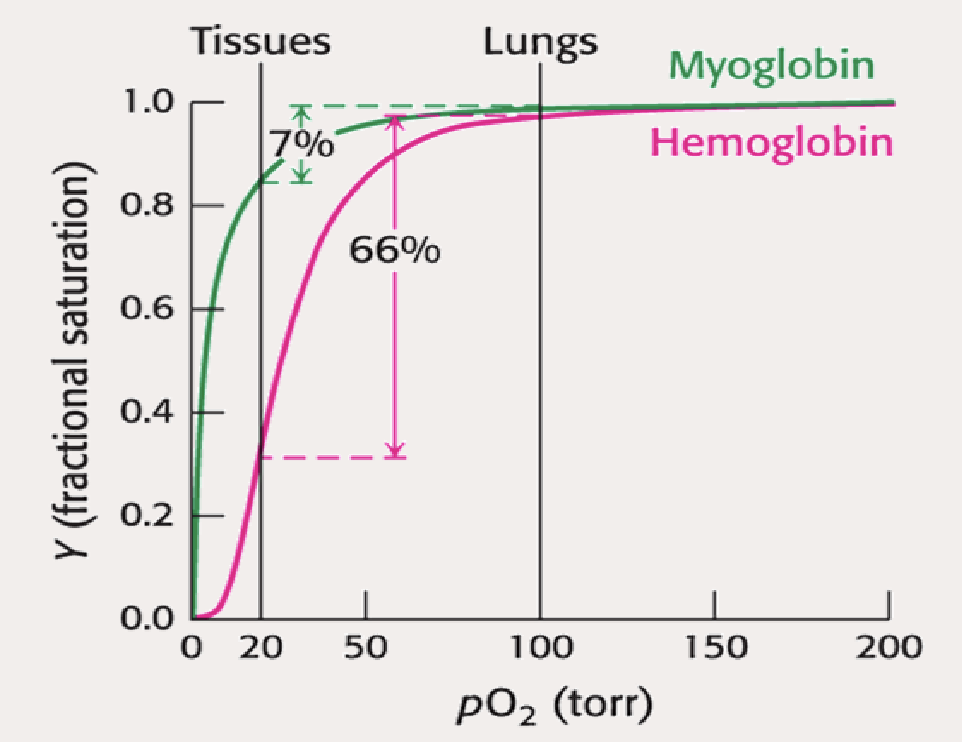
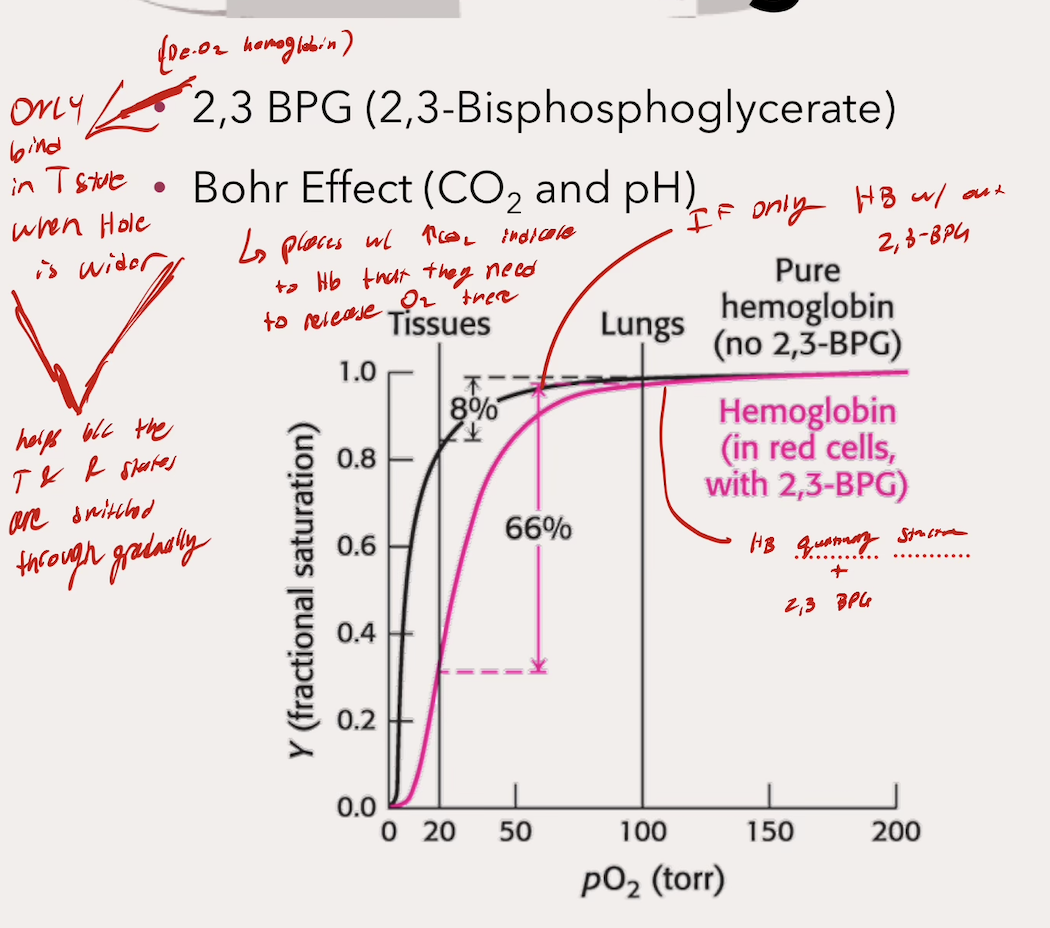
What does this curve illustrate about myoglobin and hemoglobin function? What do the percentages mean, and why (for Hb)?
Myoglobin → It’s only capable of delivering 7% of )2 it’s gained in the lungs (makes sense beacuse it is a storage molecule)
Hemoglobin → Hb releases 66% of it’s O2 gained from the lungs (makes sense beacuse it is a transporter)
Hb able to do this with Quaternary Strcutre and ALLOSTERIC REGULATION
What are the forms of Allosteric Regulation that Hb uses? Are they promoters or inhibitors? What does that mean?
These are all INHIBITORS
That means that they result in the DELIVERING (release) of O2 of Hb (which is it’s job), so overall helps the function
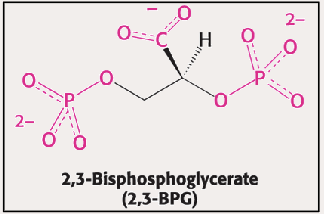
2,3 BPG
Bohr Effect
CO2
pH
What does 2,3 BPG do? Where does it bind?
Binds to Hb in the T State within the hole when it is still wide → Stabilizes the T state → INC P50 → DEC O2 affinity → Less binding → More O2 delivery
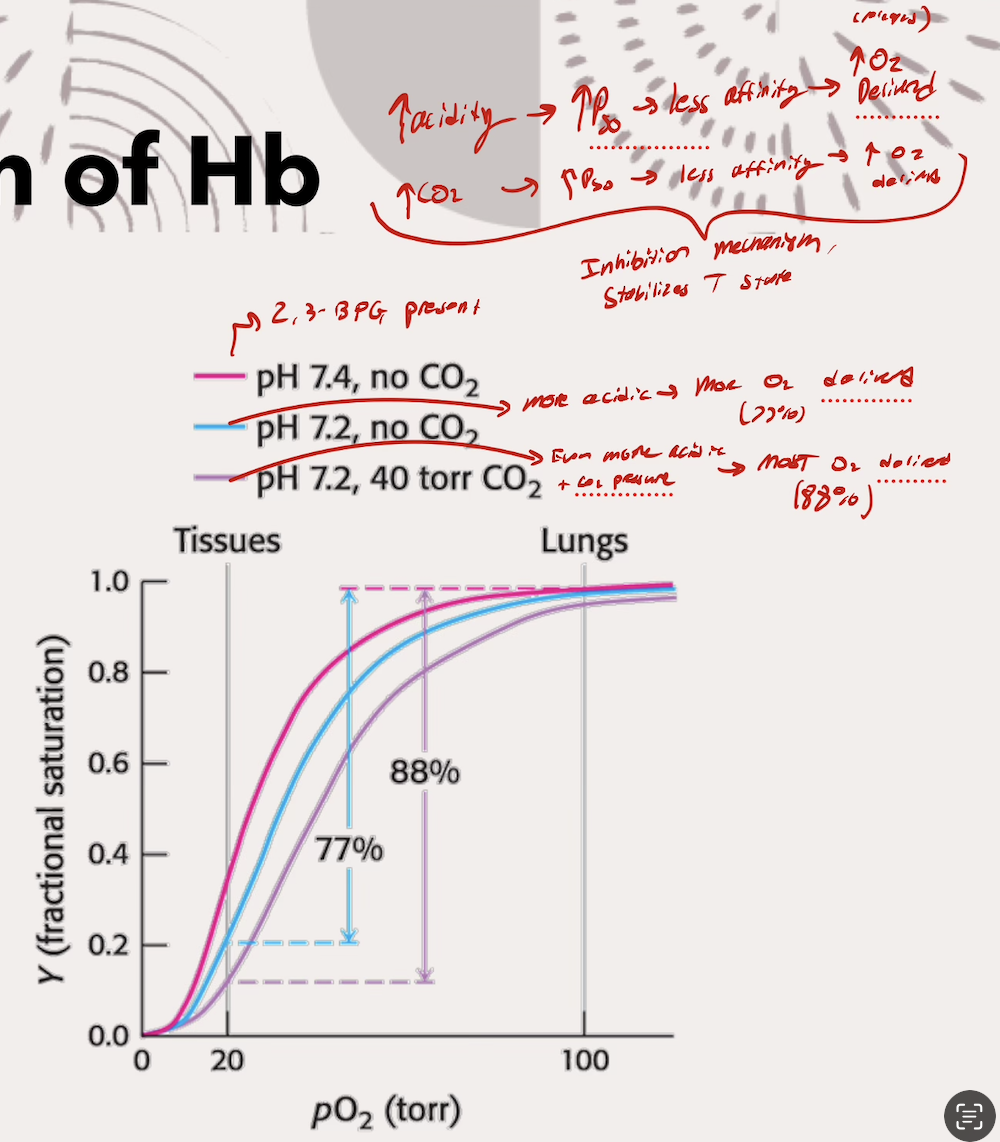
What does each part of the Bohr effect (CO2 and pH) do? What is actually happening (the mechanism)
Lower pH’s (increasing acidity) → INC P50 → Less affinity for O2 → MORE O2 delivered
Acidic environment protonates key amino acids that form ionic bonds and salt bridges
Higher pCO2 → INC P50 → Less affinity for O2 → MORE O2 delivered
CO2 binds to N-terminus (start) of eaceh Hb subunit (so four CO2 binds total), forming carbamate group
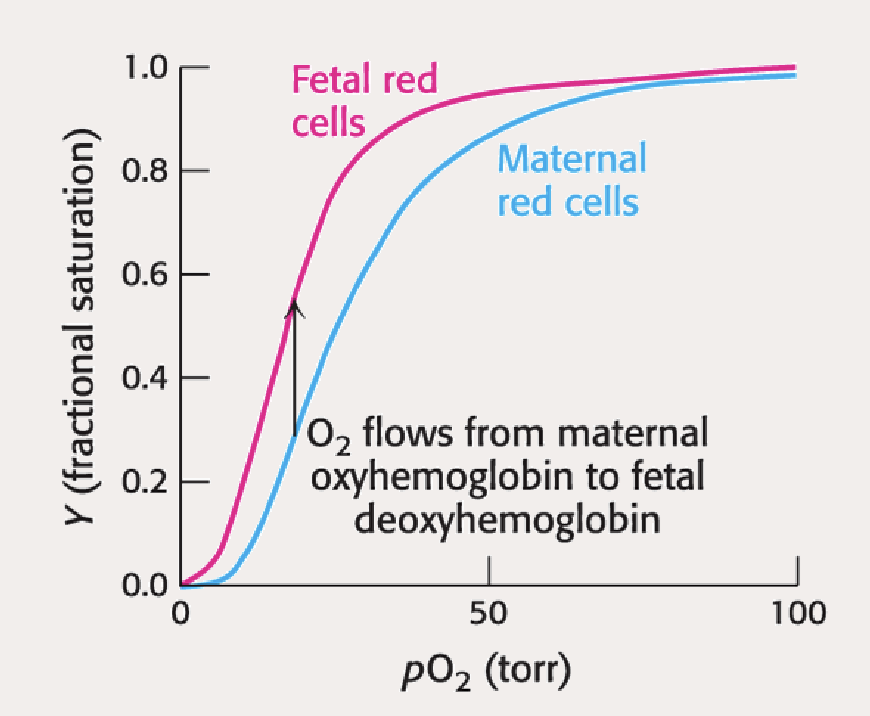
Why does fetal Hb have to have a higher affinty than the mother? What has evolution done ot address that?
Baby needs to outcompete the mother for the oxygen from the blood supply, otherwise it would have no way og getting oxygen
Mutation in 2,3 BPG allows for LESS O2 release, and MORE O2 binding (the goal of the mutation is to make 2,3 BPG useless in a way, since it would usually promote O2 release by stabilizing the T state)
What is the mutation in Hb? How doees it’s properties change? Map out what happens in relation to 2,3 BPG
Beta subunit replaced with Gamma subunit (His143Ser mutation)
Mutation causes Hb to LOSE negative charge, and GAIN a POSITIVE CHARGE
BPG has positive charge → BPG does NOT bind to the heme group hole, DEstabilizing the T state → Higher Affinity (DEC P50) for binding now driving toward stabilizing the R state
Which way would the curve shift with the mutation?
Curve shifts left, illustrating how 2,3 BPG can’t bind and interact with anything, allowing switches into the R state, increasing affinity for O2 binding
What happpens with hemoglobin in Sickle Cell Anemia? How does the Valine change the way that Hb interact with each other?
A genetic diseases caused by a Glu6Val mutation to B chains (Glutamine → Valine)
Valine exposed to deoxyHb and interacts with deoxyHb’s that aggregate and deform the red blood cells
Valine doesn’t want to be sticking out since it is nonpolar, wants to be inside something, so it binds to other DeOxy Hb binding sites
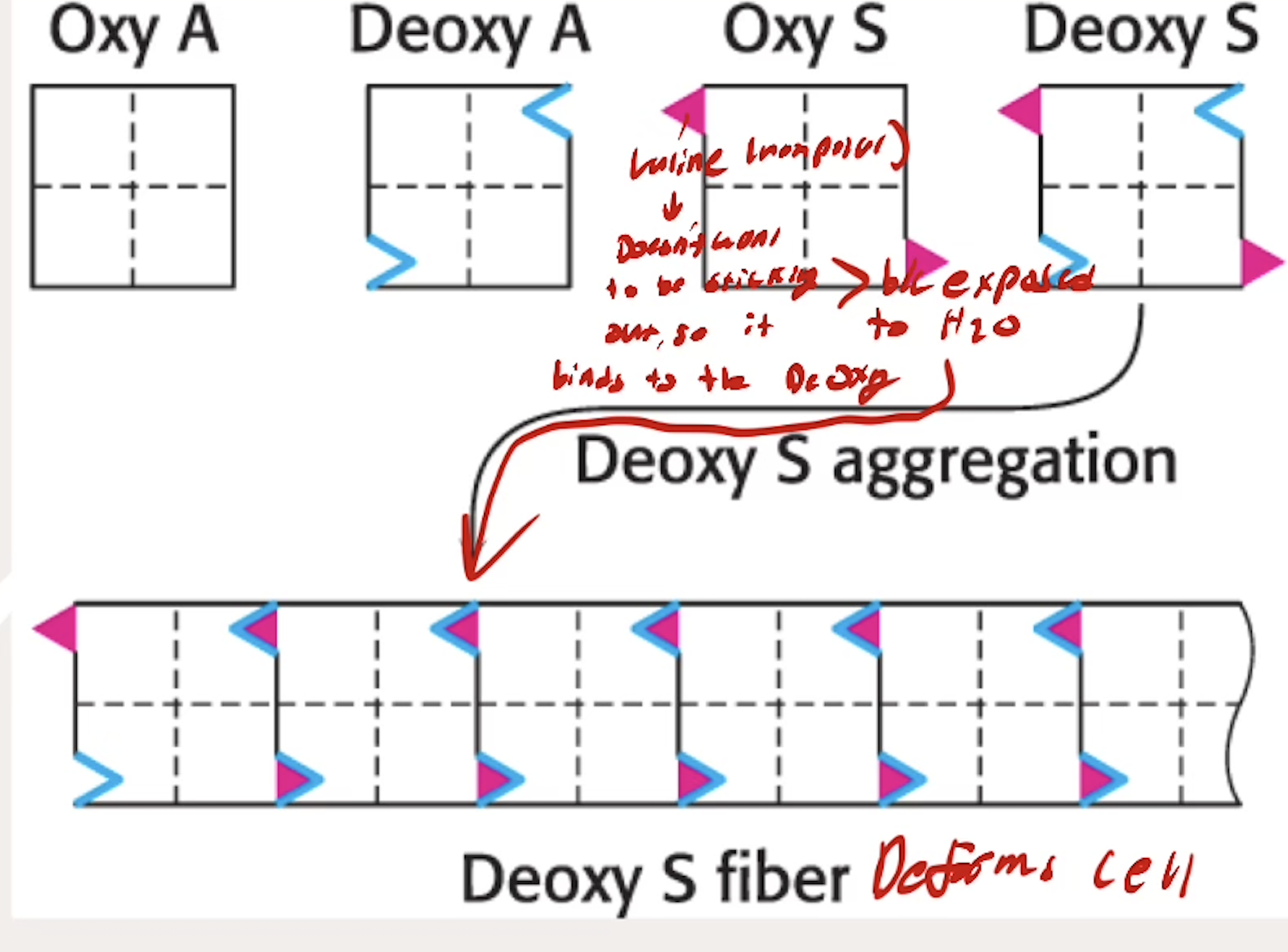
Does Sickle Cell Anemia change affinity for O2?
NO
Sickle Cell has NO AFFECT ON AFFINITY (there are less Hb overall in the first place because they are dying)