1. Electronic Structure
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
Shells
Energy levels - it increases as the shell number increases (shell number - principal quantum number n)
n = 1
2 electrons
n = 2
8 electrons
n = 3
18 electrons
n = 4
32 electrons
Formula for the maximum number of electrons in shell (n)
2n2
Atomic orbitals
A region around the nucleus that can hold up to 2 electrons with opposite spins
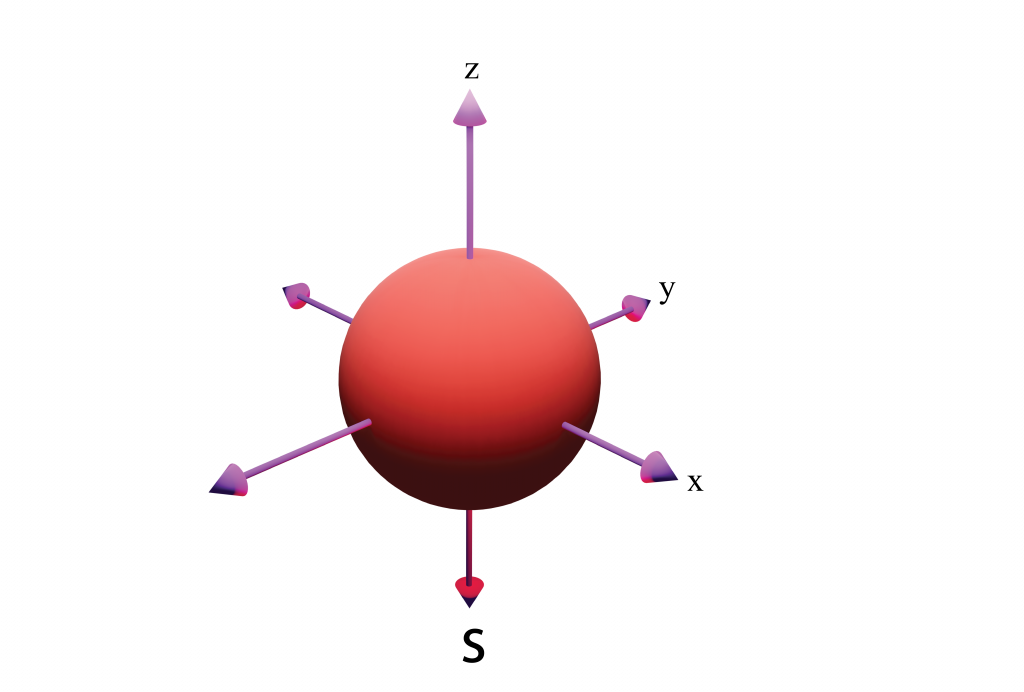
S-orbitals
Spherical shape
n = 1 has 1 s-orbital (sub-shell 2)
The greater the shell number (n), the greater the radius of the s-orbital
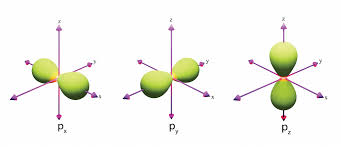
P-orbital
Dumb-bell shape
n = 2 has 3 p-orbitals (sub-shell 6)
The greater the shell number (n), the further the p-orbital is from the nucleus
D-orbital
More complex
n = 3 has 5 d-orbitals (sub-shell 10)
F-orbital
More complex
n = 4 has 7 f-orbitals (sub-shell 14)
n = 1 order of filling
1s (2)
n = 2 shell order of filling
2s (2), 2p (6)
n = 3 order of filling
3s (2), 3p (6), 3d (10)
However, 3d has a higher energy level than 4s so 4s goes first
n = 4 order of filling
4s (2), 4p (6), 4d (8), 4f (14)
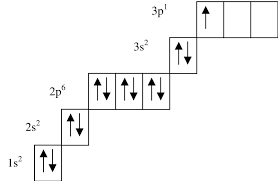
Electrons-in-box model - explanation
Electrons are negatively charged and they repel one another
They have a property called spin (up or down)
They must have opposite spins to counteract the repulsion between the negative charges
Orbitals with the same energy level
One electron has to occupy each orbital before pairing starts
This prevents any repulsion between paired electrons until there is no further orbital available
Exceptions for 3d before 4s
Copper and Chromium
Simplified version of the Electronic Configuration
Find the noble gas which fills up closest to the configuration (it must be lower)
Blocks in the Periodic Table
S-Block - group 1 and 2
P-Block - group 3, 4, 5, 6, 7, 8
D-Block - middle table