5- Kinetics
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
Why does the Maxwell-boltzmann distribution curve start at 0?
No particles have zero energy
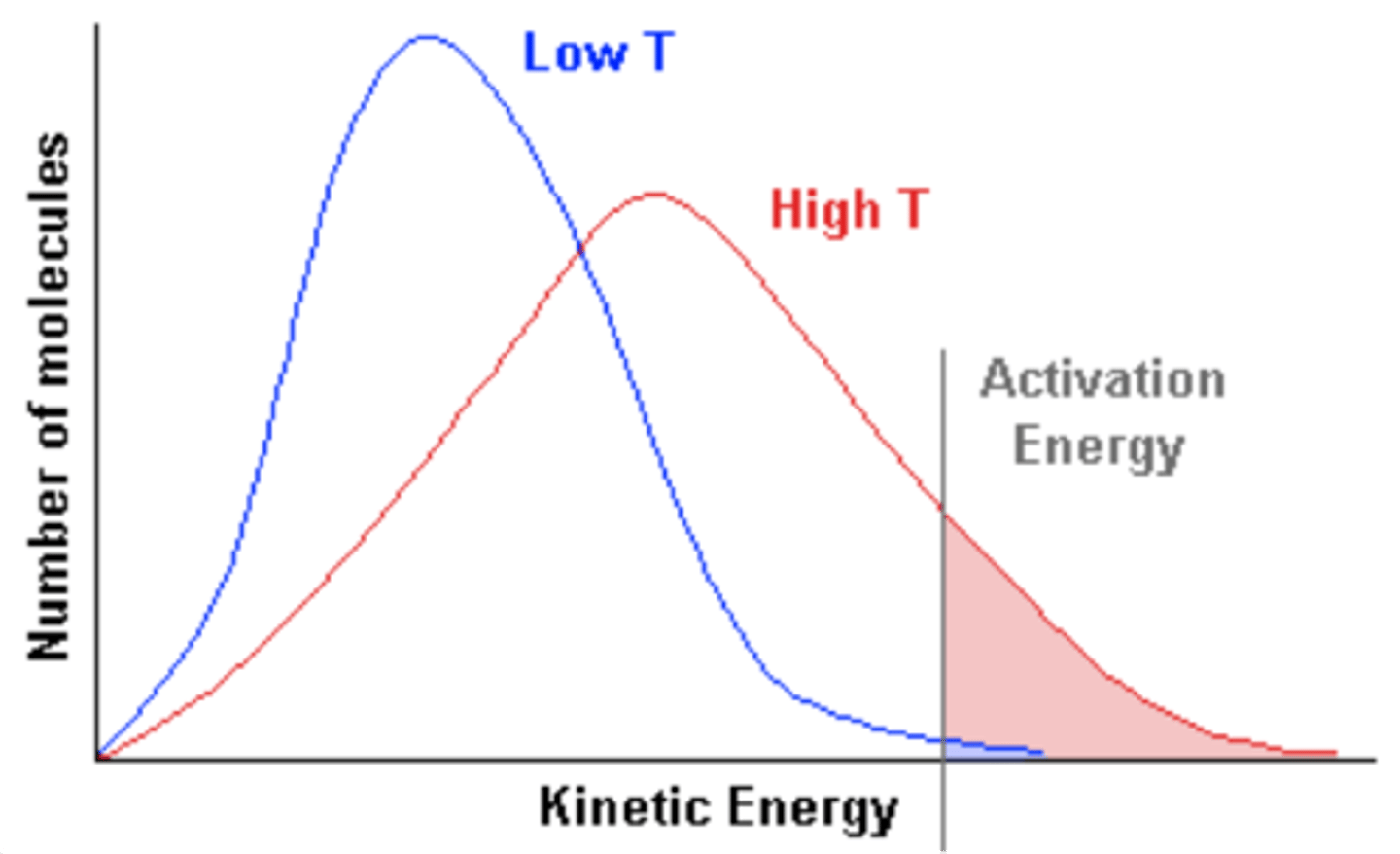
Explain the effect of increasing temperature on the curve of the Maxwell-boltzman graph
-particles have more kinetic energy
-causing particles to move around faster resulting in more frequent collisions
-proportion of successful collisions increases, meaning a higher proportion of particles possess the activation energy
-with higher temps, the Boltzmann curve flattens and shifts to the right
(opposite for a decrease in temp)
Why do not all reactions that are exothermic occur at room temperature?
Activation energy must be present.
For example, fuels are mostly safe at room temperature. But a small spark may provide enough energy to start the combustion reaction.
What does the area under the maxwell-boltzman curve tell us?
Total number of molecules in a system
Where is
a) the most probable energy on the Maxwell-boltzman graph
b) the mean energy on the Maxwell-Boltzmann graph
c) a catalyst activation energy on the graph
d) activation energy
a) directly under the peak of the curve
b) slight to the right of the most probably energy (particles with very high energy skew the mean to the right)
c) to the left of the activation energy
d) 2/3d alone the x axis
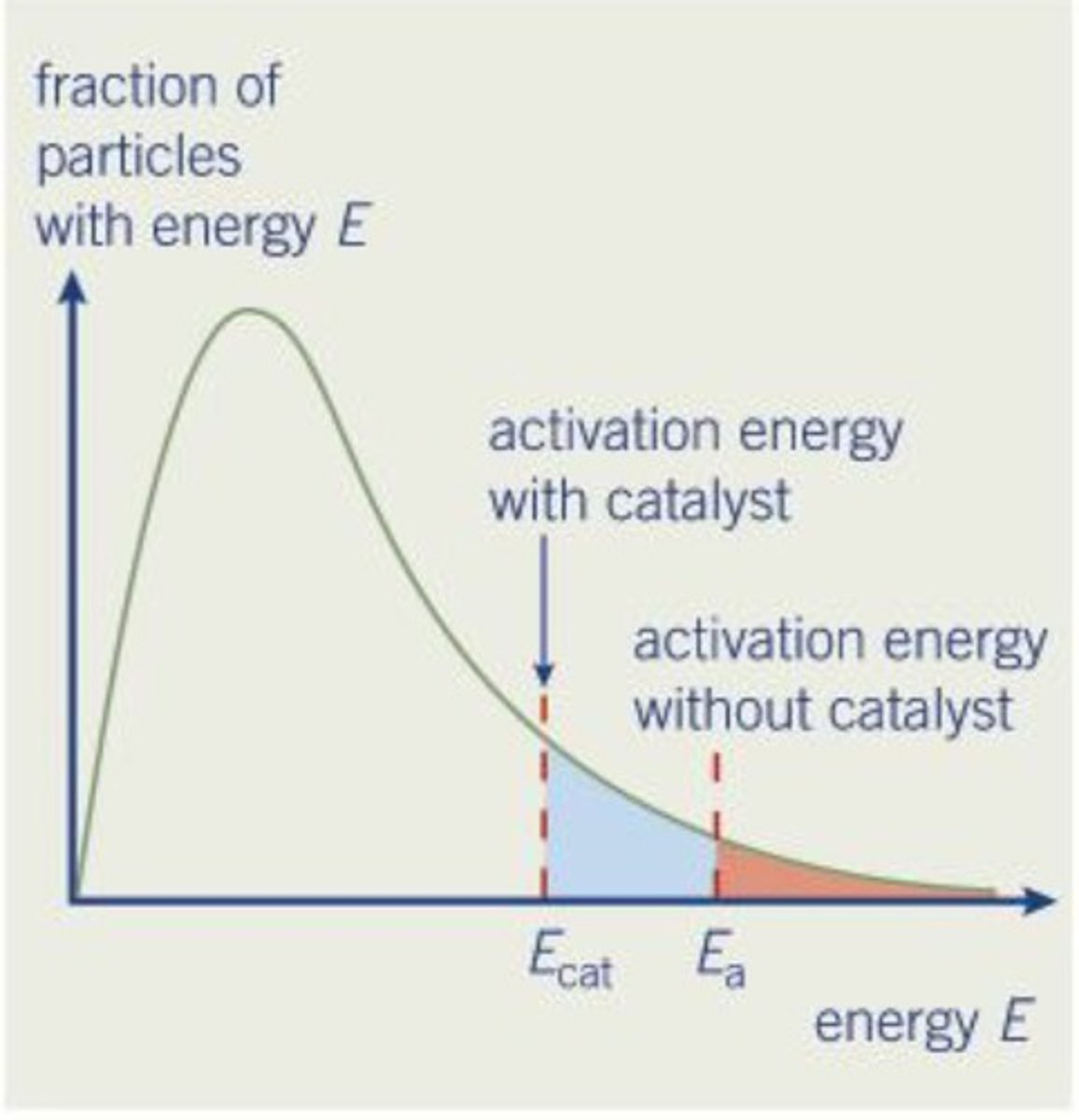
What does Ea and Ec represent
Ea - activation energy
Ec- activation energy with catalyst.
What does the Maxwell-boltzman graph represent?
Distribution of molecular energies in gasses
Explain the effect of a catalyst on the Maxwell-boltzman graph
-An alternative pathway with a lower activation energy is provided (represented by the shift of Ea to the left)
-According to the graph, using a catalyst increases the proportion of particles that have at least the activation energy
(shaded region of Ea increases)
-thus increasing the rate of reaction
Explain why most collisions don't lead to a reaction
-particles do not have enough energy
-particles are in the wrong orientation
Explain the effect of removing particles on the Maxwell-boltzman curve
Does not shift as there is no change in energy, the curve lowers slightly as the molecules are removed
State the role of water in a reaction with calcium
Oxidising agent
Explain the effect of increasing concentration on the rate of reaction
More particles in the same space, successful collisions are more frequent
Equation for rate of reaction
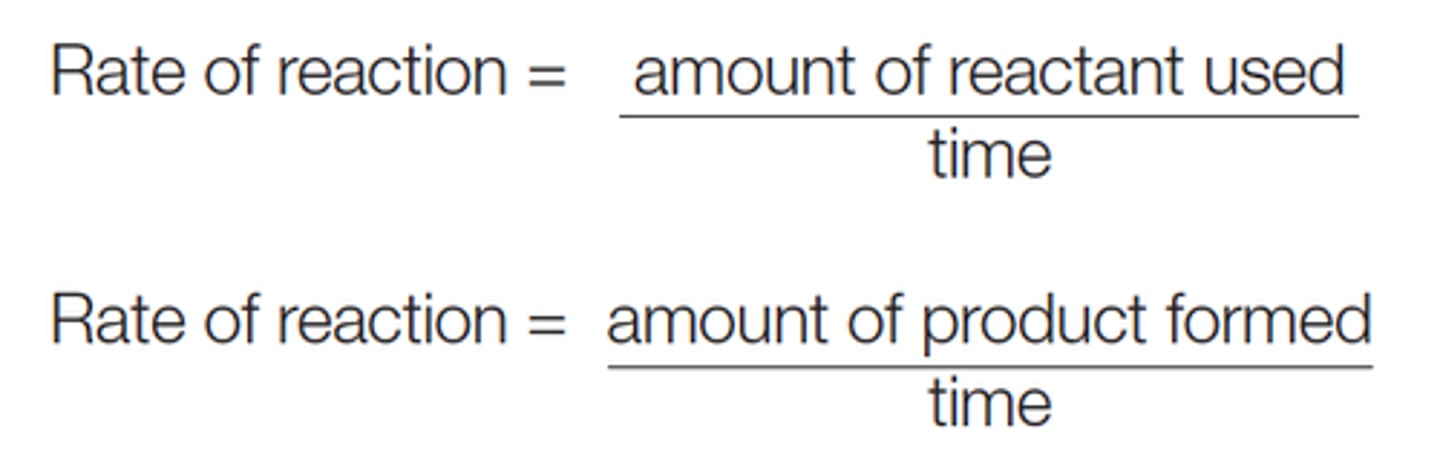
Equation for rate of reaction used