Properties of Life and Basic Chemistry Concepts
1/97
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
98 Terms
Biology
study of life
Adaptation
how an organism gets fitted to their environment; result of evolution by natural selection, a trait that allows an organism success in their environment
Order
organisms are highly organized, coordinated structures with one or more cells (organelles => cells => organs...)
Response to Stimuli
respond to diverse stimuli (think plant reaching for sunlight)
Regulation
coordinate internal functions, respond to stimuli, and cope with environmental stresses => homeostasis
Reproduction
organisms reproduce (ranging from single celled replication and division to specialized reproductive cells)
Energy Processing
use energy for metabolic processes (photosynthesis, consumption)
Evolution
random changes in hereditary material (mutations) result in characteristics that make an organism more or less fit for the environment (more fit=more reproductive success=pass on)
Homeostasis
steady state, relatively stable internal environment required to maintain life
Macromolecule
large molecules that typically formed by polymerization (polymers made up of monomers)
Atom
smallest and most fundamental unit of matter that retains properties of an element - nucleus surrounded by electrons
Molecule
chemical structure of at least 2 atoms held together by one or more chemical bond
Organelles
A mass of macromolecules surrounded by a membrane that exist within cells and serve specific functions
Cell
smallest fundamental unit of structure and function in living organisms
Prokaryote
single-celled or colonial organism; do NOT have membrane-bound nuclei
Eukaryote
DO have membrane bound organelles and membrane bound nucleus
Tissues
comprised of cells that combine and carry out similar or related functions (ex: skin cells combine to form skin tissue)
Organs
collections of tissues grouped together performing a common function
Organ system
higher level of organization consisting of functionally related organs
Organism
individual living entity
Microorganisms
single-celled prokaryotes and single-celled eukaryotes
Matter
any substance that occupies space and has mass
Elements
unique forms of matter with specific chemical and physical properties that cannot break down into smaller substances by ordinary chemical reaction
Mass number
#protons + #neutrons
Atomic number
#of Protons
Isotopes
different forms of the same atom that vary only in the number of neutrons they possess; # of neutrons varies
Atomic mass
Mean of mass number for its isotopes.
Chemical reactivity
Ability to combine and chemically bond with one another (based on # and spatial distribution of e-); combine to form molecules.
Electrons in a chemically neutral atom
electrons = # protons.
Electron shells/energy levels
Electrons fill orbitals in consistent order = lowest energy shell (1n) to highest (3n).
Octet rule
Atoms are more energetically stable when they have 8 electrons in their valence shell (outermost electron shell).
Noble gases (Group 18)
Have 8 electrons in valence shell, highly stable, don't want to share electrons (form bonds).
Group 1 elements
Have 1 valence electron, want to donate or share 1 electron to gain stability.
Group 17 elements
Have 7 valence electrons, want to fill the shell with an electron from another atom or molecule.
Electron orbitals
Where electrons are most likely to be found and they form complex shapes.
s subshell
orbitals: 1; # of electrons it holds: 2; Shape: sphere.
p subshell
orbitals: 3; # of electrons it holds: 6; Shape: dumbbell.
d subshell
orbitals: 5; # of electrons it holds: 10; Shape: complex.
f subshell
orbitals: 7; # of electrons it holds: 14; Shape: complex.
Electron configuration of H
1s1.
Electron configuration of Ne
1s2,2s2,2p6.
Electron configuration of Li
1s2,2s1.
Electron configuration of C
1s2,2s2,2p2
Electron configuration of Cl
1s2,2s2,2p6,3s2,3p5
Valence shell electrons
Determine an atom's energetic stability and its tendency to form chemical bonds with other atoms.
Electrons in innermost shell
Max of 2 electrons; atoms will fill first.
Next two shells
Max of 8 electrons.
Chemical reaction
when 2 or more atoms combine or break apart
Molecule
2 or more atoms chemically bonded
Ionic bond
form between a cation and anion to form neutral charge (transfer of electrons)
Cation
+ charge (given away electrons)
Anion
-charge (received electrons, add -ide to the name ex: sulfur to sulfide)
Ionic bond example
Na+ + Cl- = NaCl (table salt!)
Covalent bond
share electrons between atoms and are much stronger and more common than ionic bonds
Single bond
sharing 1 electron
Double bond
sharing 2 electrons
Triple bond
sharing 3 electrons
Polar covalent bond
atoms unequally share electrons, creating slightly negative (δ-) or positive charge(δ+) ex: H2O
Electronegativity
tendency of an atom to attract shared electrons
Nonpolar covalent bond
occur between two atoms of the same element or between different elements that share electrons equally ex: CH4
Hydrogen bond
weak interaction formed by slightly positive charge around hydrogen attracting neighboring negative charges
Van der Waals interactions
weak attractions between molecules dependent on slight fluctuations of electron densities around an atom
Hydrophilic
polar substance that interacts readily with or dissolves in water
Hydrophobic
nonpolar substances that do not interact readily or dissolve in water ex: fats and oils
States of water
Liquid, Gas, Solid - unique characteristics crucial to life
Liquid water
water molecules break/reform as they slide past each other due to kinetic energy
Ice
crystalline structure maintained by H bonds, decreasing density, allowing ice to float
Gas “liquid”
as temp rises with boiling, higher kinetic energy causes bonds to break completely and molecules escape (steam, water vapor)
High heat capacity
Water can absorb a lot of heat before its temperature changes.
Specific heat
The amount of heat one gram of a substance must absorb or lose to change its temperature by 1 degree Celsius (this amount of heat = 1 calorie).
High specific heat
Takes lots of heat to change the temperature of water and takes lots of time to heat and cool.
Heat of vaporization
The amount of energy required to change one gram of a liquid into a gas.
Evaporation
Below the boiling point (100℃), water's individual molecules gain enough energy from other molecules that some at the surface can escape and vaporize.
Solvent properties
Ions and polar molecules can readily dissolve in H2O, making it a solvent capable of dissolving other polar molecules and ionic compounds.
Dissociation
When ionic compounds (like NaCl) are added to water, their ions react with water molecules' polar regions and their bond is disrupted.
Sphere of hydration
Forms around the ions when they dissolve in water, with slight + or - charge attracted to ions.
Cohesion
Water molecules are attracted to each other (H bonding), keeping water molecules together at the liquid-gas (water-air) interface.
Surface tension
Capacity of a substance to withstand rupturing when placed under tension or stress; cohesion allows for surface tension.
Adhesion
Attraction between water molecules and other molecules; occurs in capillary tubes where adhesion > cohesion.
pH
Indicates the acidity or basicity of a solution on a scale.
Acid
Substance that increases the concentration of hydrogen ions (H+) in solution; stronger acids more readily donate H+.
Base
Substance that decreases the concentration of H+ in a solution, either by adding hydroxide ions (OH-) or other negatively charged ions.
Alkaline
A solution with a pH of 8 and up (14 being the most basic).
Acidic
A solution with a pH of 6 and down (1 being the most acidic).
Neutral
A solution with a pH of 7.
Buffers
Readily absorb excess H+ or OH- keeping pH consistent.
Organic molecules
Any carbon-containing liquid, solid, or gas.
Hydrocarbons
Organic molecules consisting entirely of H and C; successive bonds form hydrocarbon chains.
Isomers
Share the same chemical formula but differ in structure of atoms or bonds, resulting in differences in chemical properties.
Geometric Isomers
Isomers that differ in the arrangement of groups around a double bond.
Enantiomers
Same chemical structures and bonds, but different 3-D structure. (still mirror each other)
Functional group
Groups of atoms that occur within molecules and result in specific chemical properties.
Hydrogen bonds
Can link functional groups together, such as in DNA.
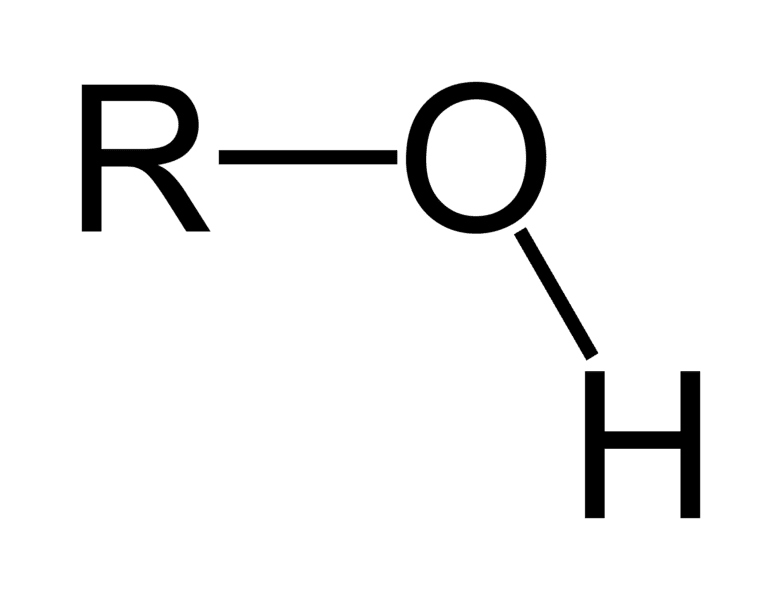
Hydroxyl group
consists of one hydrogen single-bonded to an oxygen atom (in sugars and alcohols)(polar)
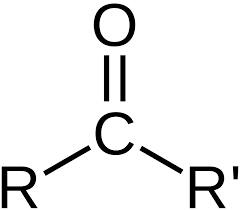
Carbonyl
consists of a carbon atom double-bonded to an oxygen atom (polar)
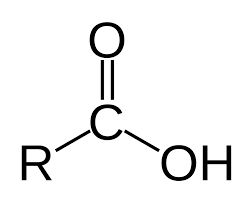
Carboxyl group
consists of a carbon double bonded to an oxygen and also singlely bonded to a hydroxyl group (OH)
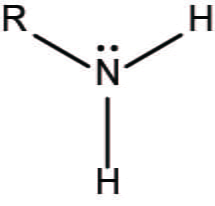
Amino group
consists of a nitrogen atom singly bonded to two hydrogen atoms (Charged; accepts H+ to form NH3+, since amino groups can remove H+ from solution, they are basic)
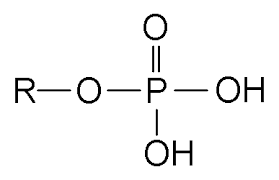
Phosphate group
consists of a phosphorus atom bonded to four oxygen atoms (3 singles and 1 double bonds) (sometimes there will be hydrogen/hydroxyl group)