Lecture 21 - bioenergetics 4 (ETC)
1/67
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
68 Terms
the majority of atp to meet requirements of eukaryotic cells comes from what source?
comes from reduced electron carriers (NADH and FADH2) that transfer their electrons to a series of membrane bound electron carriers known as the respiratory chain
when they get transfereed through membrane bound respiratory chain. what is the final electron acceptor and what does it form at the end?
The final electron acceptor is oxygen which is reduced to water.
what happens to the energy released by that process?
The energy released by this process is partially captured by the generation of ATP from ADP and Pi. Since the phosphorylation process is indirectly dependent on oxygen, this form of phosphorylation is known as oxidative phosphorylation.
where the mitochondria originate from?
The mitochondrion is thought to be an
ancient prokaryotic, aerobic bacterium
that took up symbiotic residence in a
primitive, eukaryotic, anaerobic host
what is evidence of this acient independence?
Has its own DNA, ribosomes, and tRNAs
How many membranes does mitochondria have?
They are enclosed by an outer, highly permeable membrane and an inner impermeable membrane
what is the function of the matrix in mitochondria?
contains pyruvate dehydrogenase, TCA cycle enzymes,
and other enzymes for oxidation of amino acids and fatty acids.
where is the electron transport chain located?
The ETC is embedded in the inner membrane, where it creates a proton gradient used by ATP synthase to make ATP
how are protein complexes formed in the electron transport train?
When the inner mitochondrial membrane is gently disrupted (e.g. using digitonin and detergents), it releases 5 major protein complexes.
what complexes are part of the ETC?
Complexes I through IV make up the ETC.
ATP synthase (also called Complex V) is involved in ATP production but is not part of the ETC itself.
name the four complexes?
complex 1 (NADH-Q Oxidoreductase)
complex 2 (Succinate-Q Reductase)
complex 3 (Ubiquinone: Cytochrome c Oxidoreductase)
complex 4 (Cytochrome c Oxidase)
key info about complex 1?
Contains FMN and Fe-S clusters; 43 subunits
Complex I is the largest and accepts electrons from NADH.
key info about complex 2?
Uses FAD; only 4 subunits
Complex II is part of the TCA cycle and accepts electrons from FADH₂.
Complex II does not pump protons.
key info about complex 3? (2)
Cytochromes b and c₁; 11 subunits
Complexes III and IV are cytochrome complexes, involved in passing electrons and pumping protons.
key info about complex 4?
Cytochromes a and a₃; 13 subunits
Complexes III and IV are cytochrome complexes, involved in passing electrons and pumping protons.
Only Complexes I, III, and IV pump protons.
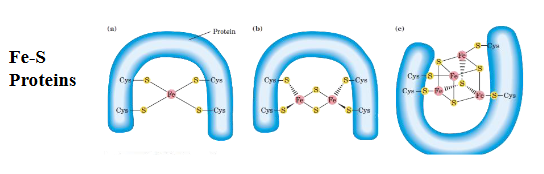
what are Fe-S Proteins (Iron-Sulfur Proteins)?
These are electron transfer cofactors found in complexes I, II, and III of the ETC.
They consist of iron (Fe) and sulfur (S) atoms coordinated by cysteine residues (a sulfur-containing amino acid) from the protein.
what is their function?
Function: They accept and donate electrons by cycling between reduced and oxidized states of iron (Fe²⁺ ↔ Fe³⁺).
Their electronic structure and arrangement define how effectively they can transfer electrons down the ETC.
what are Respirasome?
Normally, ETC complexes are shown separately, but in reality, Complexes I, III, and IV are physically associated into a "supercomplex" known as the respirasome.
Cytochrome c only binds the respirasome when it's actively transferring electro
what is the benefit of forming a respirasome (binding complexes into super complexes) (3)
Faster and more efficient electron flow.
Prevents diffusion loss or damage (like reactive oxygen species).
Enhances coordination of electron transfer steps.
properties of Ubiquinone (Q) (location, type, e source)
Type: Lipid-soluble electron carrier.
Location: Stays in the inner mitochondrial membrane because of its long hydrophobic tail (hydrocarbon chain).
Electron Sources: Accepts electrons from Complex I or II and transfers them to Complex III.
function of Ubiquinone (Q
Accepts 2 electrons and 2 protons to become QH₂ (reduced form).
properties of Cytochrome c (location, type, e source)
Type: Water-soluble electron carrier (floats in the intermembrane space)
Contains: A heme group with iron that switches between:
Fe³⁺ (oxidized) → Fe²⁺ (reduced).
Location: Loosely associated on the outer surface of the inner mitochondrial membrane.
function of cytochrome c?
Transfers 1 electron at a time from Complex III to Complex IV.
how is the standard reduction potential related to these electron carriers?
Since the different components of the electron transport chain have different affinities for electrons (standard reduction potential), we can predict the sequence of electron flow down the chain to oxygen.
reduction potential of the major electron carriers

what is the flow of electrons?
Electrons released from NADH (E°' = –0.32 V)
Passed down through Complex I → Q → Complex III → cytochrome c → Complex IV → O₂ (E°' = +0.82 V)
what happens to free energy as electrons drop through each step?
∆G°' becomes more negative → energy is released
This energy is used to pump protons (H⁺) across the inner mitochondrial membrane
function of pmf?
ATP synthesis via ATP synthase (next step)
Electron & Proton Flow Through the ETC

how many protons are pumped out of the
matrix into the intermembrane space for every two electrons transferred from NADH to
oxygen?
Complex | H⁺ Pumped per 2 e⁻ |
|---|---|
Complex I | 4 |
Complex II | 0 |
Complex III | 4 |
Complex IV | 2 |
Total (NADH) | 10 H⁺ |
Total (FADH₂) | 6 H⁺ (skips Complex I) |
why are only 6 protons translocated during their passage through the ETC to O2
The two electrons from the oxidation of FADH2 do not
pass through complex I, therefore only 6 protons are
translocated during their passage through the ETC to O
how much energy is required to pump 1 mole of protons across the inner mitochondrial membrane
Energy required to pump 1 mole of H⁺ = 19.4 kJ
10 moles of protons (per 2 e⁻ from NADH):
→ 19.4 kJ/mol × 10 mol = 194 kJ
what is the free energy change for the transfer of electrons from nadh to oxygen?
ΔG°’ for NADH → O₂ = −220 kJ/mol
→ This means NADH oxidation releases enough energy to:
Pump the 10 H⁺ across the membrane
And still leave a surplus of ~26 kJ for other processes.
Main Point from all of this?
The oxidation of NADH has more than enough energy to drive proton pumping across the membrane — setting up the proton-motive force that will drive ATP synthesis.
Proton translocation across the inner mitochondrial membrane results in two key gradients name them
Charge separation
Positive (H⁺) outside (intermembrane space)
Negative inside (matrix)
Proton concentration gradient
Higher [H⁺] in the intermembrane space
Lower [H⁺] in the matrix
These gradients together make up the proton motive force (pmf), a form of stored electrochemical energy.
what is the structure of complex V
Complex V is made of two main parts:
1. F₀ (membrane-embedded portion)
F₁ (matrix-facing portion)
what is the F₀ (membrane-embedded portion)
Located in the inner mitochondrial membrane
Formed of 8–14 c subunits (arranged in a ring)
Functions as a proton channel
what is the F₁ (matrix-facing portion)?
Extends into the mitochondrial matrix
Composed of:
3 α and 3 β subunits (forms the α₃β₃ hexamer)
γ, δ, and ε subunits (form the central stalk)
The γ subunit rotates in the center of the α₃β₃ hexamer, driven by proton movement.
How does proton flow leads to ATP synthesis:
Protons (H⁺) re-enter the matrix through the F₀ c subunits
Each proton enters a c subunit channel, causing the c ring to rotate
This rotor motion is transferred to the γ subunit, which spins inside the α₃β₃ complex
what does the rotation to the y subunit cause?
Rotation of the γ within the α3β3 hexamer induces
conformational changes in the hexamer called open (O), loose (L) and tight (T). These different conformations are used to generate ATP from ADP and Pi
How Rotation Leads to ATP Formation?
The γ subunit sits in the center and rotates 120° steps due to proton flow through the c ring.
This rotation causes each of the 3 β subunits to cycle through O → L → T → O conformations sequentially.
explain how ATP synthase (Complex V) synthesizes ATP using the proton motive force (pmf)
The open conformation ADP + Pi can access
the nucleotide binding sitePassage of protons through the transmembrane c subunits causes the γ subunit to rotate 120⁰, this forces the yellow β subunit to adopt the loose conformation.
The loose conformation results in a tighter
binding of ADP and Pi to the enzymeAs protons continue to pass through the
rotor its rotation in the membrane again forces
γ to rotate 120⁰ with the result that the yellow β subunit adopts the tight conformation. In the
tight conformation ATP is formed from the ADP
and Pi that have been sitting in the active site.A final 120⁰ rotation of γ restores the yellow
subunit to the open conformation. This
releases ATP and allows for another ADP and Pi to bind
whats happening in each step
Step | Description | What it Represents |
|---|---|---|
1⃣ | Open conformation (O): ADP + Pi bind to the yellow β subunit | Start of ATP synthesis |
2⃣ | γ subunit rotates 120°, shifting β to loose (L) state | Energy from proton flow causes this rotation |
3⃣ | Loose conformation tightens into tight (T) → ADP + Pi are now closely held | Prepares for ATP formation |
4⃣ | Another 120° γ rotation → β becomes tight and synthesizes ATP | ATP is chemically formed |
5⃣ | Final 120° γ rotation returns β to open (O) → ATP is released | Cycle restarts with new ADP + Pi |
How Many ATP Molecules Are Made per Rotation
Each full 360° rotation of the γ subunit causes all three β subunits to go through the cycle: Open → Loose → Tight.
So, 1 full rotation = 3 ATP molecules synthesized.
how many rotations can atp synthase do per second?
ATP synthase can do 10 full rotations per second, giving a maximum of 30 ATP/sec per enzyme.
On average, it takes 3 protons (H⁺) to make 1 ATP, so ~9 protons are used per full rotation.
but what is The Problem of Transport:
ATP is mostly used outside the mitochondria, but it's made inside.
ADP and Pi need to get into the mitochondria, and ATP needs to get out.
But: All three molecules are charged, and the inner mitochondrial membrane is impermeable to charged species.
what is the solution to that problem?
ATP-ADP Translocase
This is a specialized transporter in the inner membrane.
It exchanges ATP for ADP — it sends ATP out of the mitochondria and brings ADP in.
This is essential for continuous ATP production and use.
aside from atp-adp translocase what is another transporter that exports atp out?
Phosphate Carrier brings in Pi in exchange for OH⁻ (or sometimes with H⁺ depending on context).
Transporting ADP, Pi, and ATP costs energy, approximately equal to the pumping of 1 proton.
ATP Yield of the ETC
Energy cost per 1 ATP made:
Function | Protons required |
|---|---|
Rotate ATP synthase (Complex V) | 3 |
Transport ADP, ATP, Pi | 1 |
Total | 4 protons |
ATP Yield per Electron Carrier:
NADH pumps 10 protons →
10 protons ÷ 4 protons per ATP = 2.5 ATPFADH₂ pumps 6 protons (enters ETC at Complex II) →
6 protons ÷ 4 protons per ATP = 1.5 ATP
what two processes are coupled together?
ETC and ATP Synthesis
so what does that mean?
ETC (electron flow) and ATP synthesis depend on each other.
If ETC stops, no proton gradient (proton motive force, pmf) is created → no ATP synthesis.
If ATP synthesis stops, the pmf builds up too much → electron flow through the ETC stops due to backpressure.
examples of that
Cyanide inhibits Complex IV → ETC halts → ATP synthesis halts.
No oxygen → no terminal electron acceptor → ETC halts → ATP synthesis halts.
what are 3 ways you can block atp synthesis
Blocking ATP synthesis by:
defective ATP-ADP translocase
damage to ATP synthase
oligomycin (an ATPase inhibitor)
what is the consequence of no atp synthesis?
ETC shuts down because as concentration of protons in
intermembrane space gets too high the energy needed to pump them out exceeds the energy liberated by NADH
oxidation
what happens if you Uncoupling ETC and ATP synthesis?
BUT, if the ETC is uncoupled from ATP synthesis, electrons can flow down the ETC without the generation of ATP. This happens if protons pumped into intermembrane space have an alternate path (i.e. not thru ATP synthase) back into the matrix
what are uncouplers?
are agents that make it possible for protons to
return to the mitochondria matrix without passing thru the ATP synthase.
Types of uncouplers:
Small, lipid-soluble molecules (e.g., DNP)
Protein uncouplers (like UCP1)
what happens if protons return to the matrix without passing through the ATP synthase?
none of the potential energy inherent in the pmf is
captured as mechanical or chemical energy. This is known as a “proton leak”
The energy therefore is dissipated as heat. This is not necessarily wasteful
what is BAT?
brown adipose tissue
Unlike white adipose tissue, BAT store very small
amounts of lipid and it contains a large amount
mitochondria that have a protein called uncoupling
protein 1 (UCP1) or thermogenin. (thermo –heat;
genin—to create)
what does this protein (UCP1) form?
This protein forms a channel in the inner mitochondrial membrane that provides an alternate path for protons to return to the matrix.
Allows protons to leak back into the matrix without ATP production, releasing energy as heat instead.
The protein that allows protons to bypass ATP synthase and generate heat is called UCP1 (Uncoupling Protein 1), also known as thermogenin.
This is useful in?
Newborns and hibernating animals who need to stay warm.
It provides a non-shivering thermogenesis mechanism to maintain body temperature.
how resting mammals use the protein leak or ETC uncoupling to their advantage?
In resting mammals, ~20% of the proton motive force (pmf) is used not for ATP synthesis, but instead dissipated as heat via proton leaks.
It’s a natural process to regulate body temperature, especially in cold environments
Uncoupling and Weight Loss
Normally, food energy is converted to NADH & FADH₂, which generate ATP via the ETC.
When uncoupling increases:
Less ATP is made from the same amount of NADH/FADH₂.
The body compensates by burning more fuel (like fat).
This leads to weight loss, even with the same food intake.
Historical context:
n the 1930s, synthetic uncouplers (e.g., DNP) were used as weight loss drugs.
They worked — but dangerously. Many patients died from overheating, because too much energy was lost as heat instead of making ATP.
where is BAT located in humans?
In infants, BAT is found between the shoulder blades, neck, and along the spine.
In adults, smaller amounts persist in the neck, upper chest, and around the kidneys.
what does low BAT activity mean?
Low BAT activity correlates with weight gain, and BAT levels decline with age.
If we could artificially increase BAT activity, it might be used to prevent weight gain or promote weight loss.
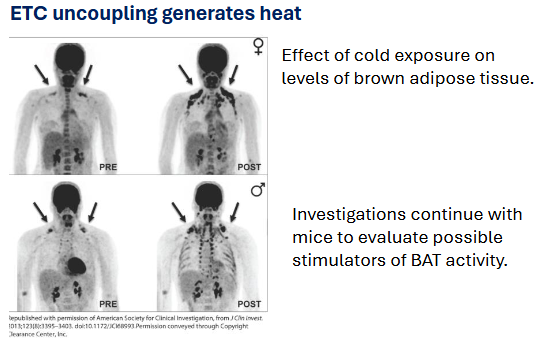
how does cold environment affect BAT lvls?
Cold exposure stimulates BAT activity (arrows show regions with higher metabolic activity).
Research continues using mice models to explore ways to stimulate BAT activity — possibly leading to safer metabolic interventions.