4: electronic spectroscopy & photochemistry
1/147
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
148 Terms
describe observable differences between atomic and molecular spectroscopy
atomic = lines observed in spectra
molecular = bands observed in spectra
define spectroscopy
investigation and measurement of spectra produced when matter interacts with or emits electromagnetic radiation (light)
describe absorption in atomic spectroscopy
there are discrete atomic energy levels. a transition from the ground state to the excited state can be initiated by incident light of the exact energy/wavelength. this can give information on the size of the energy gap and position of energy levels.
describe emission in atomic spectroscopy
there are discrete atomic energy levels. relaxation of matter from excited state to ground state emits light of the exact energy/wavelength of transition. this can give information on the size of the energy gap and position of energy levels.
define laser light
highly focused = beam
monochromatic = all same wavelength
coherent = all in phase
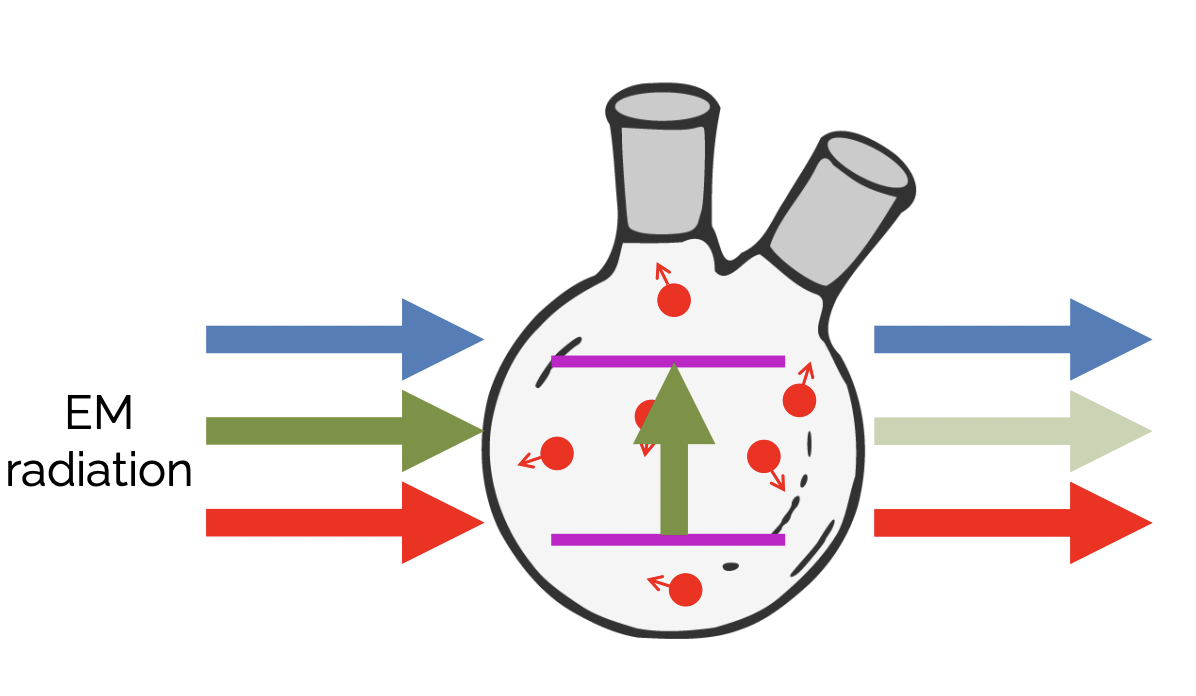
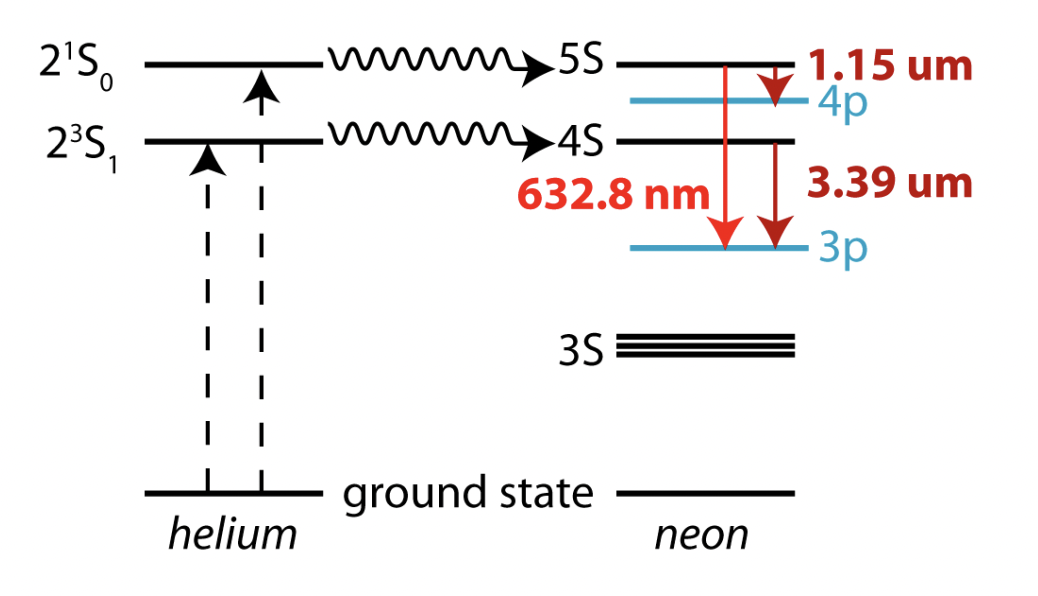
describe a possible basis for a laser
helium-neon gas mixture
helium atom is excited. this energy is transferred to a neighbouring neon atom through collisional energy transfer. this excites electrons in the neon atom. relaxation of these electrons to lower energy level emits a photon. this gives red light which is:
monochromatic = all same wavelength
coherent = all in phase
describe molecular spectroscopy
as opposed to atomic orbitals, transition occur between molecular orbitals.
what processes occur on the femtosecond timescale?
bond formation and breakage
chemical reactions
isomerisation
molecule relaxation
what different units can be used to depict energies and transitions?
wavenumber (v~)
electron volt (eV)
describe eV
e = charge of an electron = 1.6×10(-19)
divide the energy by e to yield the energy in eV
= energy gained across a potential difference of 1 volt
what is a simple method to convert from wavelength to eV?
*WAVELENGTH MUST BE IN nm*
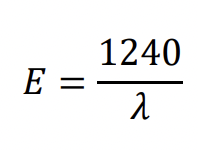
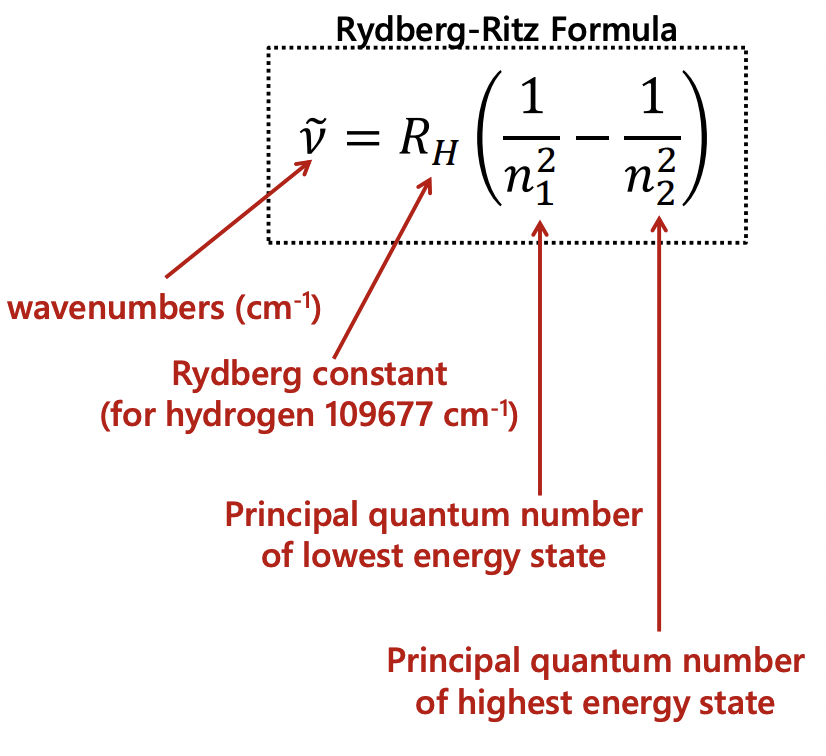
describe the Rydberg-Ritz formula
= for hydrogen = atomic
gives the wavelengths/frequencies of the spectral lines/transitions in atomic emission between quantised energy levels of the hydrogen atom
what is the Rydberg-Ritz formula?
describe the different types of transitions described by the Rydberg-Ritz formula
dependent on where the electron transition ends = lower energy level defines which series
Lyman: transition to n=1
Balmer: transition to n=2
Paschen: transition to n=3
Brackett: transition to n=4
LBPB
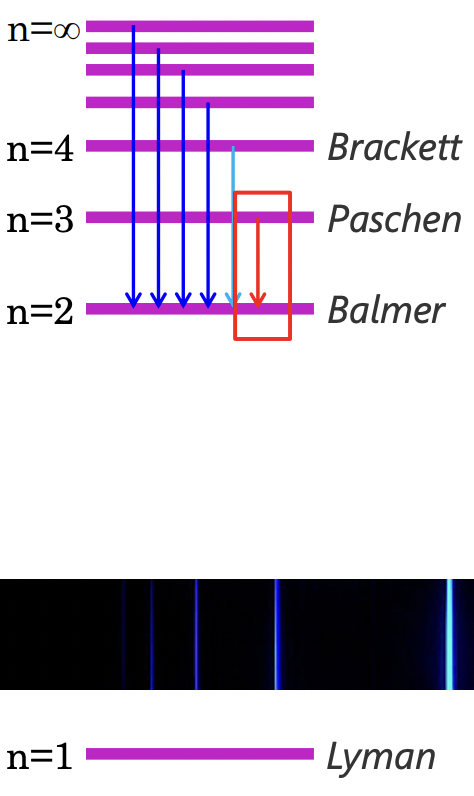
how is each transition of each type of transition defined?
nth Lyman/Balmer/Paschen?Brackett line
n = number of energy levels removed from where transition ends
i.e. 1st Lyman line: n=2 → n=1
2nd Lyman line: n=3 → n=1
1st Balmer line: n=3 → n=2
2nd Balmer line: n=4 → n=2
how does energy and wavelength vary by nth X line?
1st = smallest energy gap = largest wavelength
energy gap will increase = wavelength will decrease
describe the Balmer lines
all the transitions (nth Balmer lines) have wavelengths within the visible region

determine the wavelength of the third line in the lyman series in eV
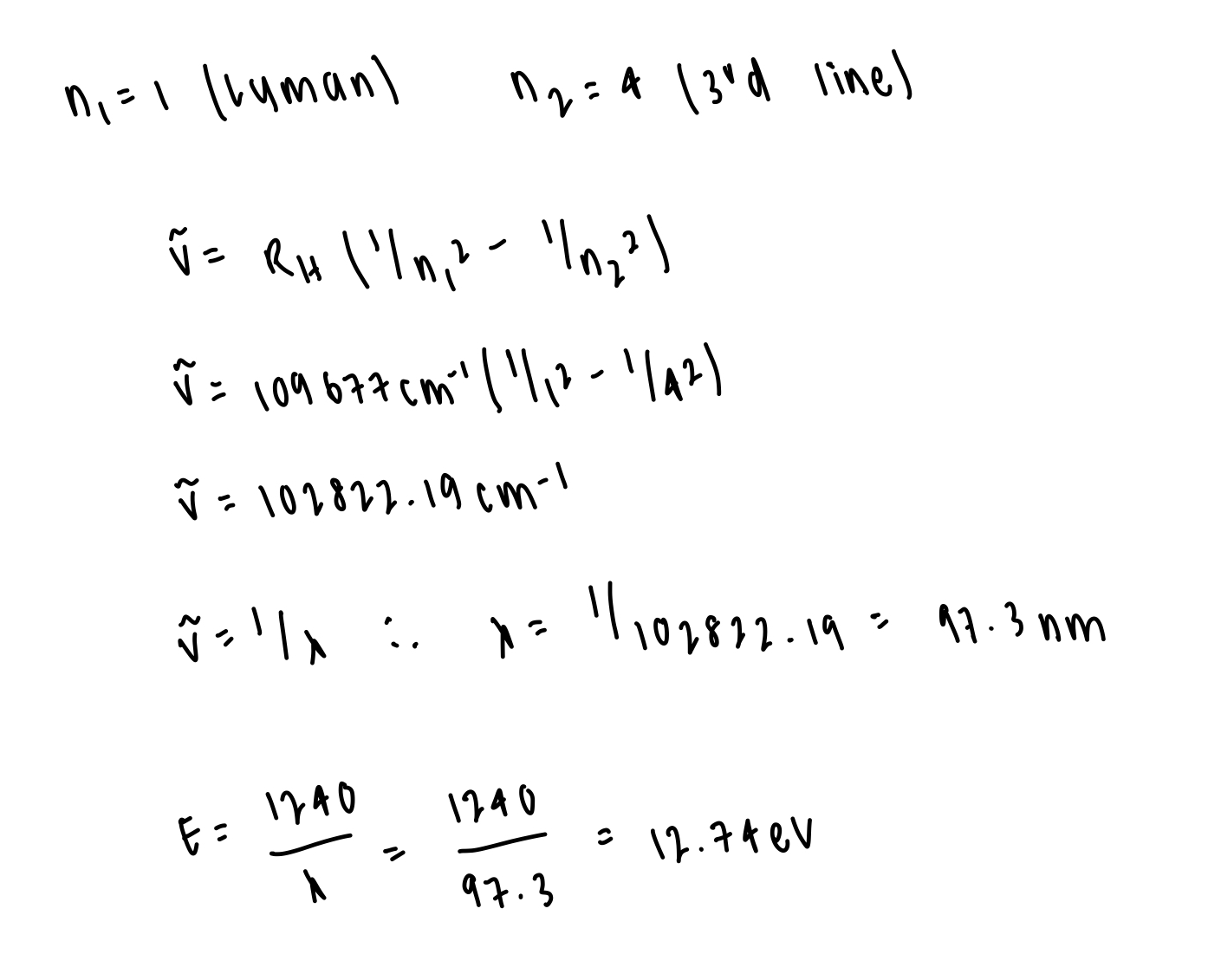
describe quantum number l
= orbital angular momentum quantum number
= different sub-shells that an electron can occupy (s, p, d) have different geometry and have different boundary conditions of the Schrodinger equation
what values can l take for each value of n? what geometries correspond to each value of l?
l = 0, 1, 2, …, (n-1)
n possible values of l for each value of n
l = 0: s
l = 1: p
l = 2: d
l = 3: f
degenerate
describe quantum number m(l)
= magnetic quantum number
= while l describes the shape of the orbital, m(l) describes the orientation of the orbitals
= the z-component of l
what values can m(l) take for each value of l?
m(l) = -l, -l+1, …, +l
2l+1 possible values of m(l) for each value of l
degenerate states
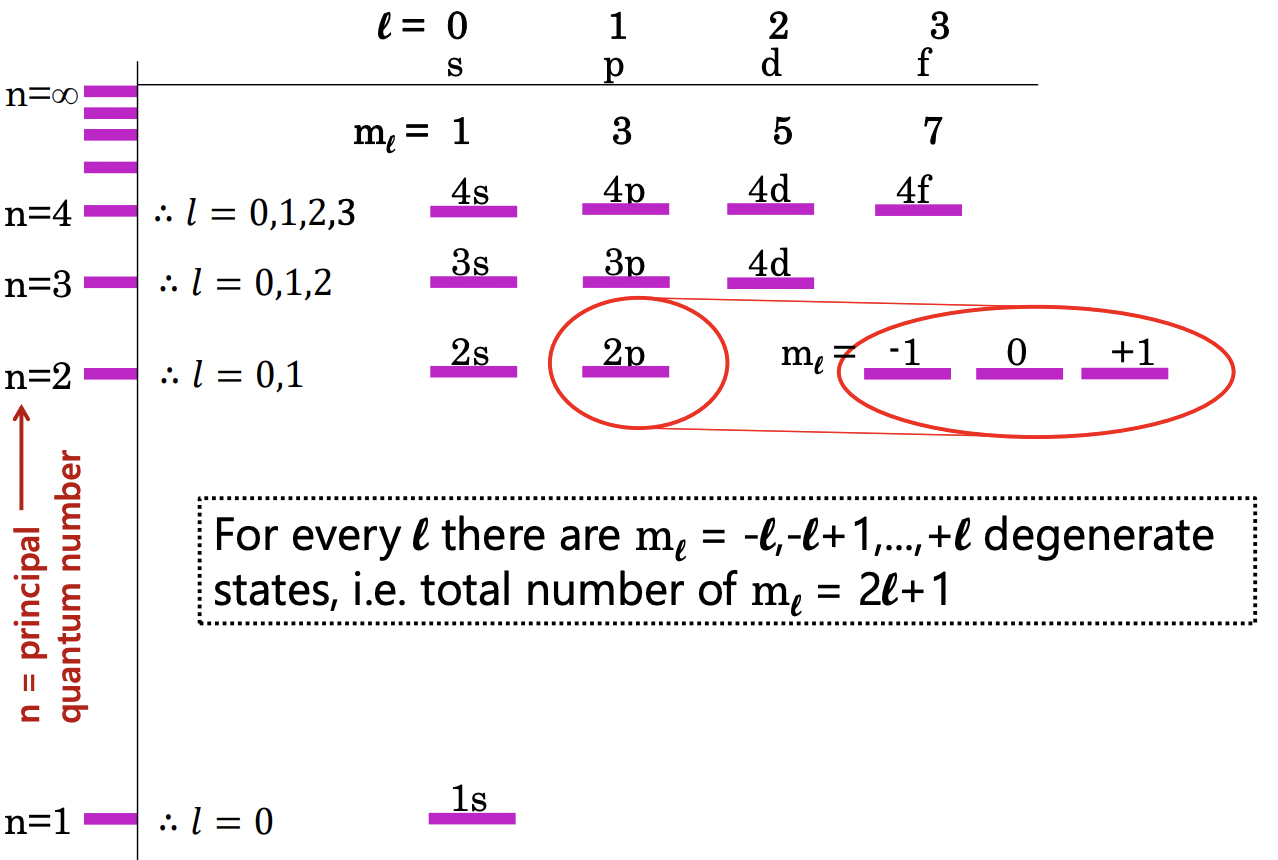
what happens to the electron states upon application of a magnetic field?
the m(l) substates lose their degeneracy
(since different orientations of orbitals will interact differently with magnetic field)
what are the selection rules for electron transitions in atomic spectrocopy?
Δl = +/- 1
Δm(l) = 0 or +/- 1
arise from conservation of angular momentum, considering photon itself has angular momentum
how can the energies of energy states in hydrogen be determined?
(in P1 section of equation sheet)
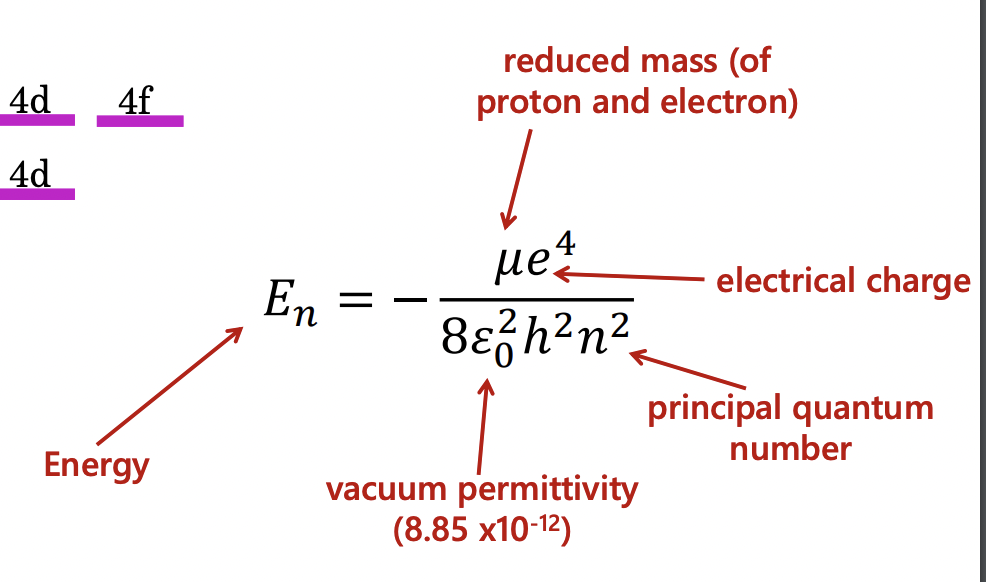
why is l not included in the energy state equation?
hydrogen is a single electron system
= energy determine only be the distance of the electron from the nucleus (Coulomb potential)
= energy NOT determined by the shape or orientation of the orbitals
this is not the same of multi-electron atoms
determine the energy level of n=1 in hydrogen atom
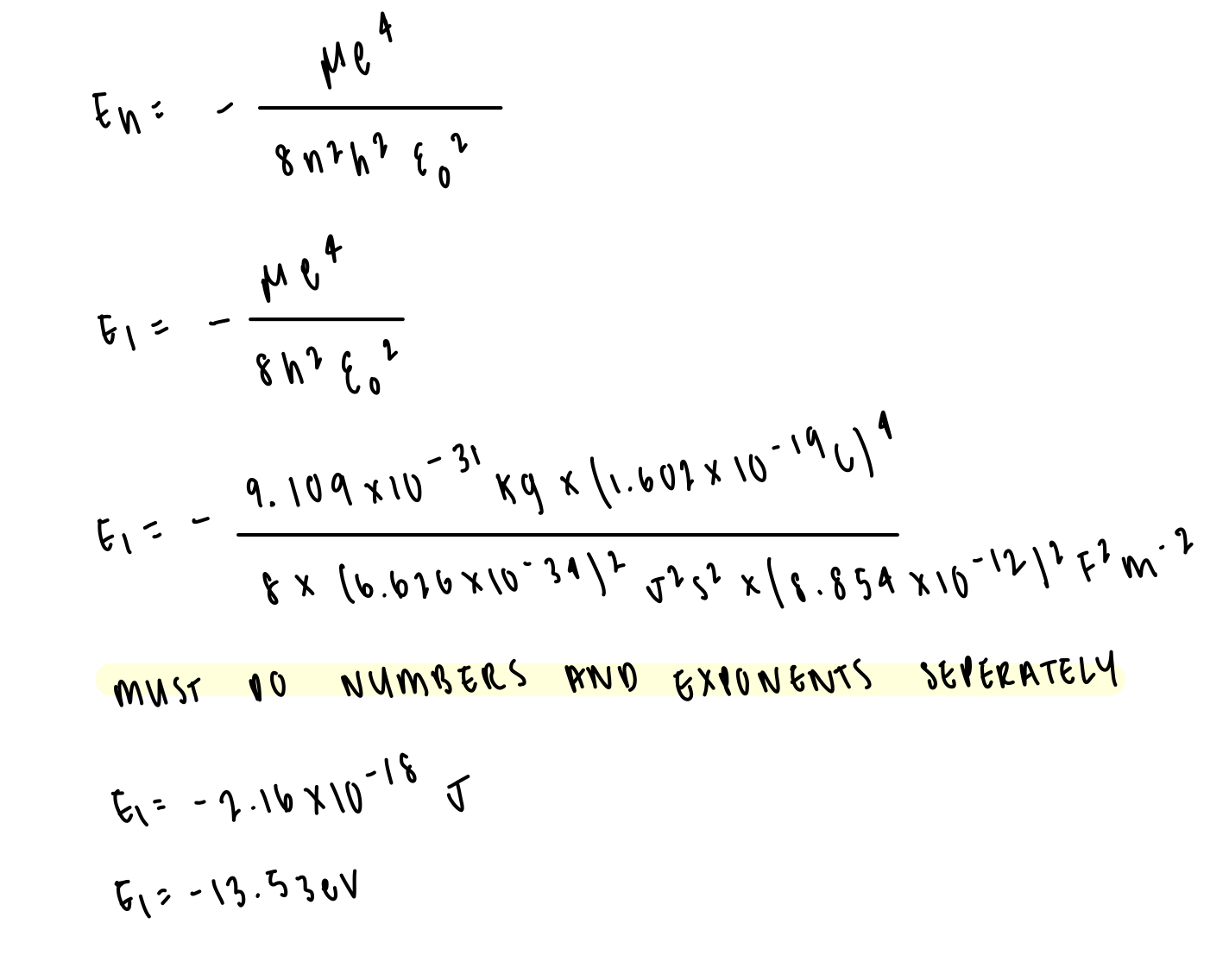
determine the energy level of n=3 in hydrogen atom
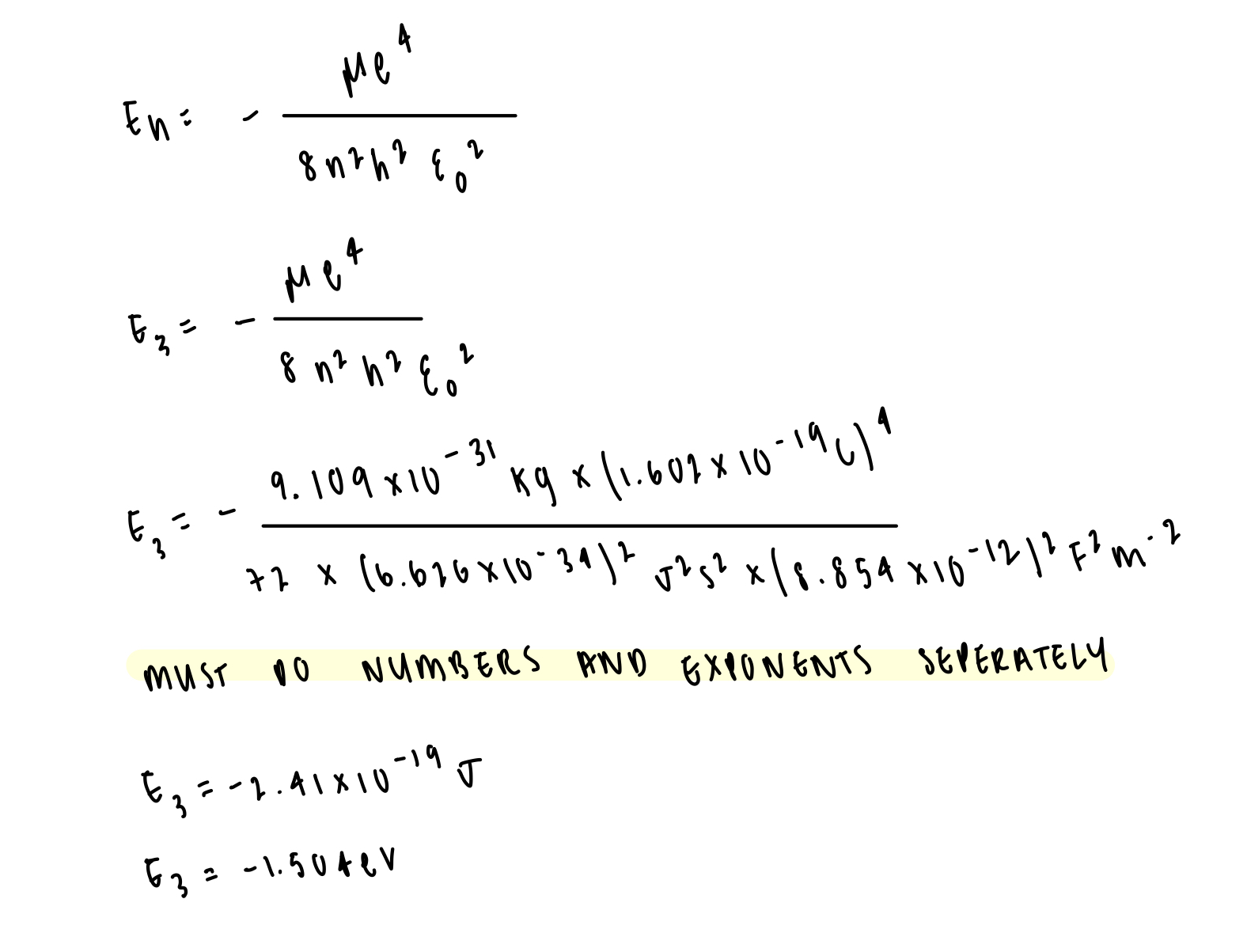
determine the size of the energy gap between n=3 and n=1 in hydrogen atom
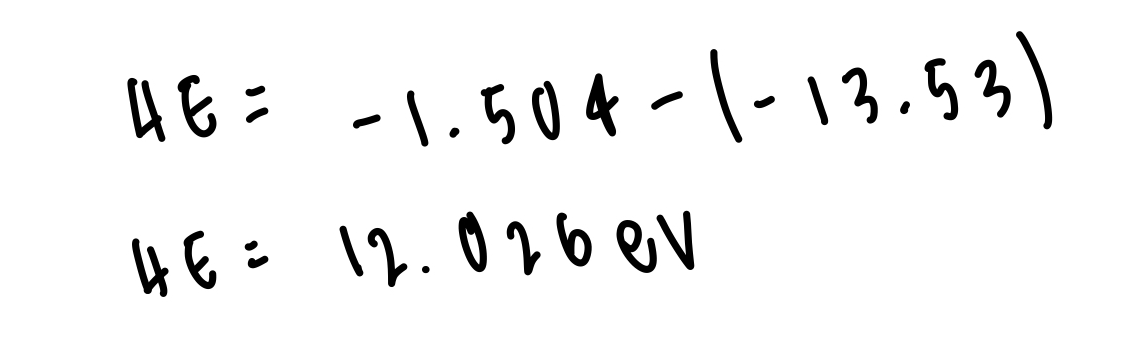
describe absorption/emission transitions in many electron atoms
single electron transitions
many transition observed for many electron atoms are typically one electron movement between orbitals, while the others generally remain in their initial states
i.e.
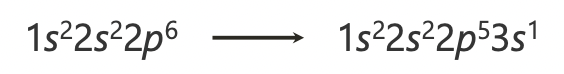
how does the atomic orbital diagram differ from hydrogen for many electron atoms?
the degeneracy of orbitals with the same value of n but different value of l is broken
why is the degeneracy of orbitals broken in many electron atoms?
spin-orbit coupling
relativistic effects
external fields
penetration
describe penetration
atoms from lithium (Z=3) and higher, their filled shells that should cancel some charge from the nucleus and ‘shield’ the outer electrons
however, the spatial probability distribution of outer electrons leaves some distribution close to the nucleus. this give rise to penetration and a larger effective nuclear charge.
greater nuclear charge = lower energy
lower values of l have greater penetrations = greater effective nuclear charge = lower energy
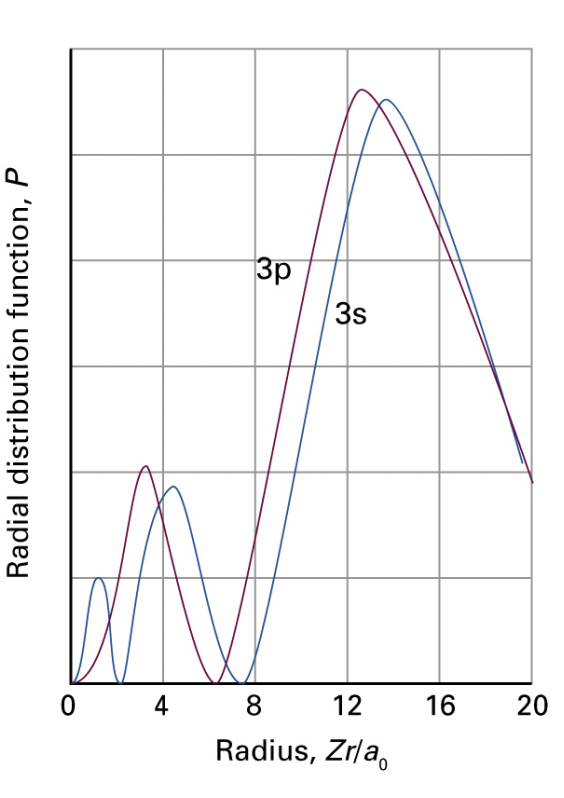
describe the relative energies of different values of l in many-electron atoms
energy: high values of l > low values of l
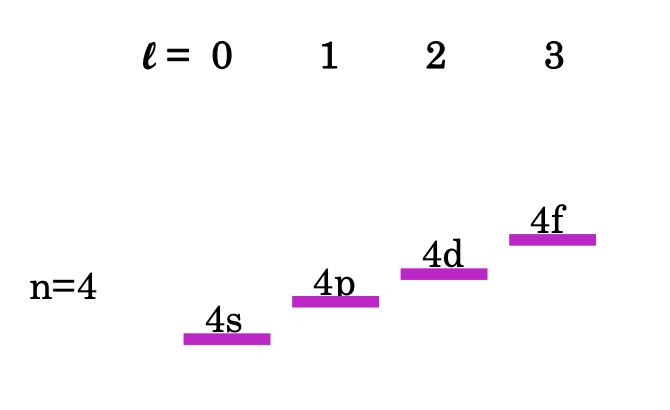
why does the Schrodinger equation not hold for many electron atoms?
interactions between electrons is to complex to be accounted for in the Schrodinger equation
what is Hund’s rule
= maximum multiplicity
= electrons will maximise spins in state = fill degenerate orbitals before coupling
what is the Pauli exclusion principle?
no two electrons can have the exact same quantum numbers
= two electrons occupying the same orbital must have opposite spins
describe quantum number s
= spin quantum number
= every electron has s = 1/2
describe quantum number m(s)
= magnetic spin quantum number
= while s describes the spin, m(s) describes the orientation of the spin
= z-component of s
= can be represented as a vector
what values can m(s) take for each value of s?
m(s) = -s, -s+1, …, +s
for electrons where s = ½
m(s) = +1/2, -1/2
what is the multiplicity of spin states
the number of different possible spin orientation combinations in a particular electronic configuration
multiplicity = 2S + 1
what is big S in the multiplicity equation?
= total spin
= sum of the m(s) values for an electronic configuration
what are the two multiplicities possible for an electron state
triplet
singlet
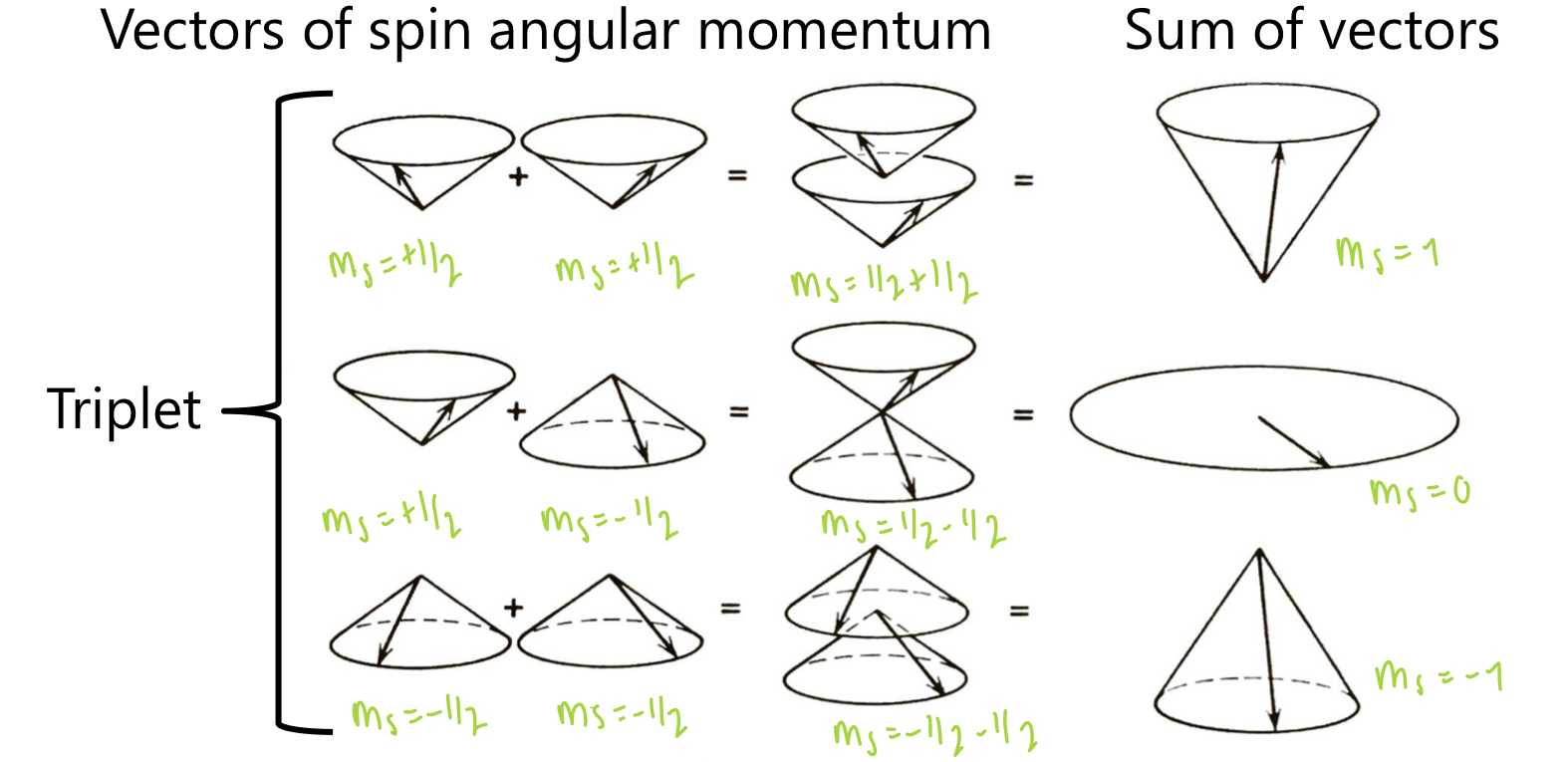
describe the triplet state
occurs when 2 electrons are unpaired
2 unpaired electrons in the ground state have the same m(s) (Hund’s rule).
hence,
S = +1/2 + +1/2 = 1
multiplicity = 2(1/2) + 1 = 3
2 unpaired electrons can have the same m(s), opposite m(s), or opposing m(s). summing the vector quantities gives 3 possible m(s) values.
describe the singlet state
occurs when 2 electrons are paired
2 paired electrons in the ground state have opposing m(s) (Pauli exclusion principle).
hence,
S = +1/2 + (- 1/2) = 0
multiplicity = 2(0) + 1 = 1
2 paired electrons have only have opposing spins. summing the vector quantities gives only 1 possible m(s) value.
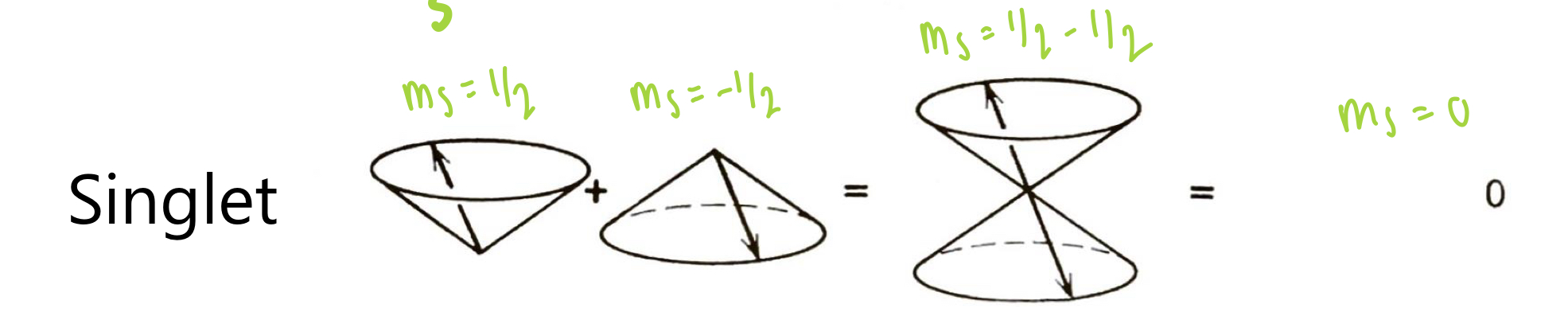
describe the decay of triplet states
the Pauli exclusion principle excludes the decay of triplet states to singlet states as a spin forbidden transition
poor to no light emission from this transition

compare spin and orbital angular momentum
spin angular momentum (s)
= rotation of the electron itself
orbital angular momentum (l)
= rotation of the electron around a point (nucleus)
describe the relative directions of spin and orbital angular momentum
spins can either be parallel or anti-parallel (vary by spin angular momentum direction ±1/2)
describe spin-orbit coupling/LS coupling/Russell-Saunders coupling
the total angular momentum can be described by quantum number j
j is the sum of spin (s) and orbital (l) quantum numbers
upon application of external field anti-parallel to l:
higher j = more resistance to external field = high E
lower j = less resistance to external field = low E
this changes the effect of the external field depending on m(s) and breaks the degeneracy of m(s) states upon application of an external field
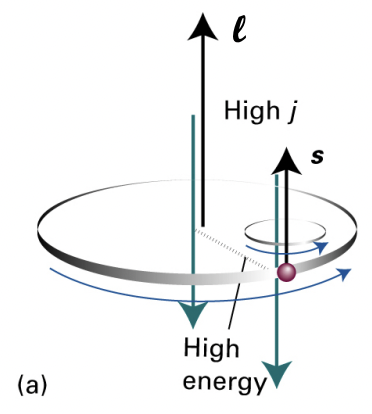
describe the value of j with the value of s
s = -1/2: anti-parallel: low j = LOWER ENERGY SUBSTATE
s = +1/2: parallel: high j = HIGHER ENERGY SUBSTATE

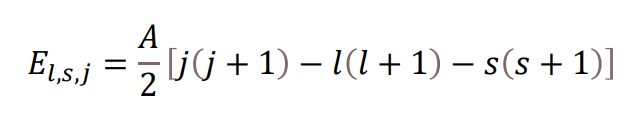
what is this equation describing and what is A?
the energy levels of substates due to spin-orbit coupling
A = spin-orbit coupling constant
A ∝ Z^4
the extent of SO splitting is much more significant for larger atoms
what effect does SO splitting have on the energy level diagram
each l state by m(s)
m(s) states are split by SO coupling upon application of external field
what are term symbols?
describe the possible states for an electronic configuration
what term is used for each state?
describes only the possible configurations of 2 unpaired electrons
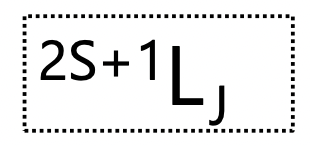
in what case is the term (1)S used?
closed shells
closed shells do not contribute angular momentum
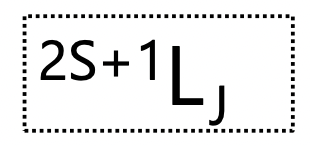
what is the ‘big L’ in the term
for two non-equivalent electrons in an unfilled outer shell
l1, l2 = l values of the configuration = can be the same OR different
(in case of 1 unpaired electrons: l1 = X, l2 = 0)
this will produce a series of L values giving the term symbols
L = 0: S
L = 1: P
L = 2: D
L = 3: F
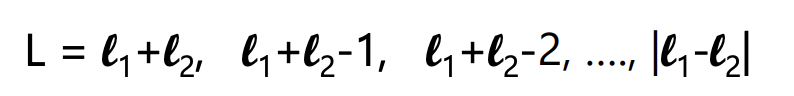
what is the ‘big S’ in the term
this yields two ~constant values:
each electron has s = ½
S = 0; S = 1
multiplicities: 1 (singlet) and 3 (triplet)
VARIES FOR SINGLE ELECTRON CONFIGURATIONS
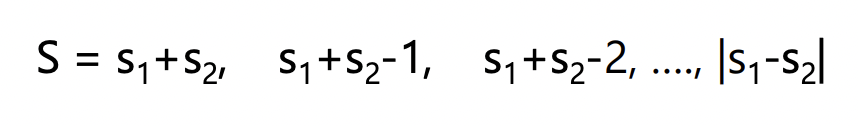
what is the ‘big J’ in the term
for a single electron:
J = j (j = l ± ½ )
for multiple electrons:
ALWAYS USE S NOT MULTIPLICITY
what do the resultant terms mean?
the terms represent possible substates
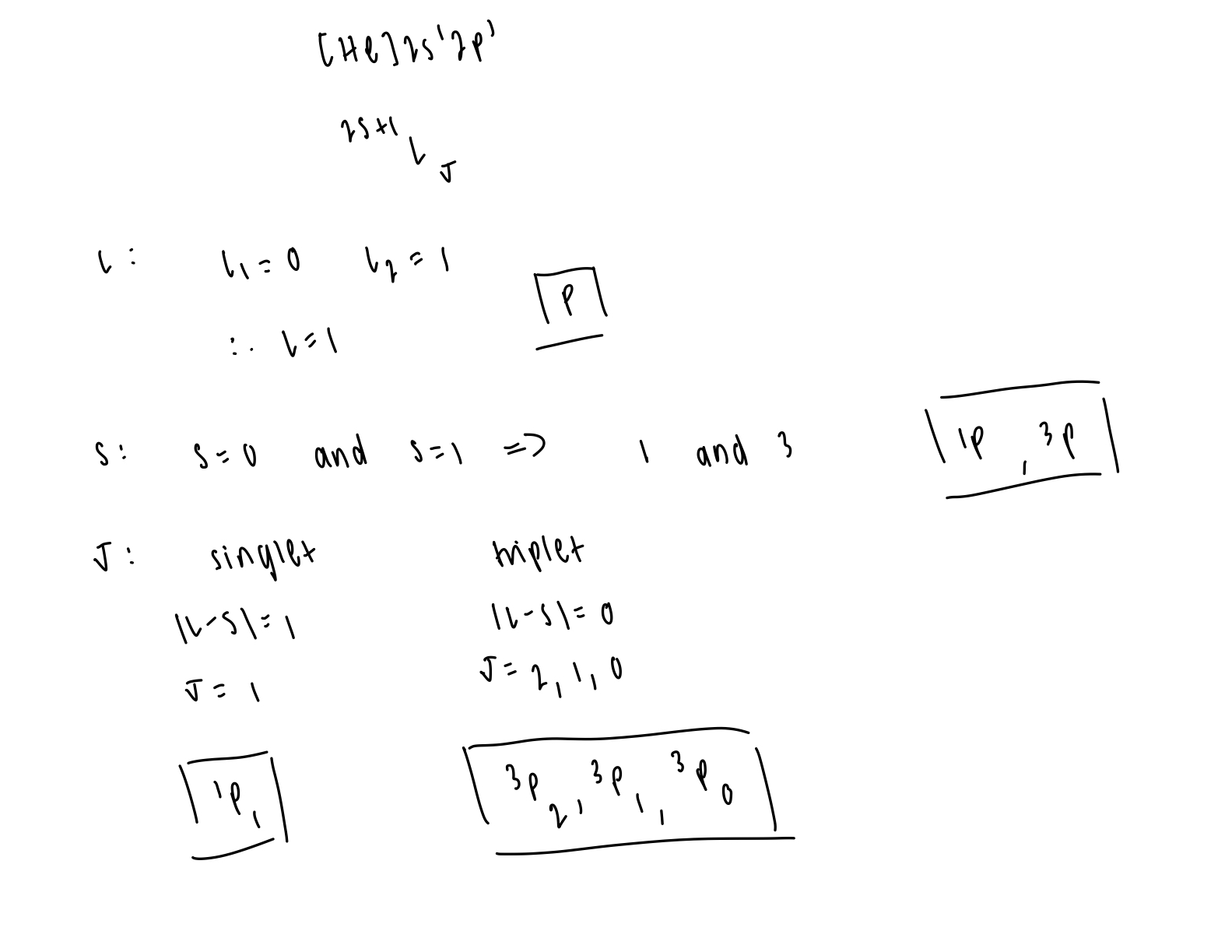
determine the possible terms and levels of [He]2s12p1
determine the possible terms and levels of partially filled shells of 2p13p1
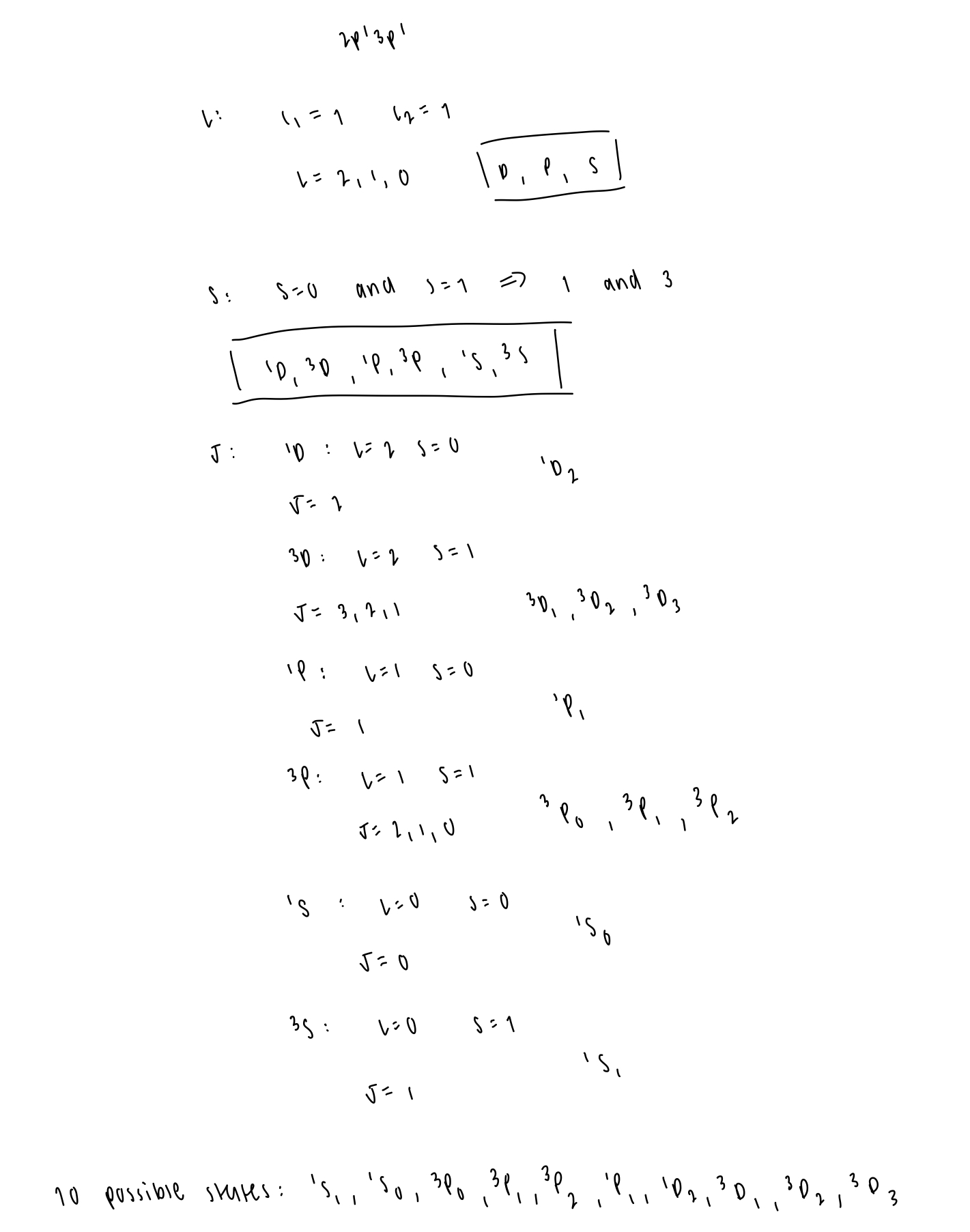
what is terms vs levels?
term = multiplicity and L value i.e. (3)P
level = multiplicity, L value AND J value i.e. (3)P(1)
describe holes
when there is a single gap in the configuration relative to closed shell, this can be treated as a single particle
Cl = 3p(5) ~ 3p(1)
determine the possible terms and levels of chlorine

what determines whether an electronic transition will occur?
selection rules
population of states
describe population of states and the probability of emission/absorption
emission = requires population of excited state
absorption = requires population of ground state
what gives rise to linewidth in atomic spectra?
lifetime broadening
Doppler broadening
atomic collisions
describe lifetime broadening
τ = time in state
longer time in state = lower δE - more known E
shorter time in state = higher δE - less known E = broader peak = excited states
only when time is infinite would we perfectly know E
anything that reduces a states lifetime increasing its lifetime broadening
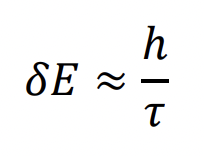
describe natural linewidth
related to the upper-state lifetime (lifetime of both upper and lower states)
broadening always present in absent of other broadening effects
rate of spontaneous emission cannot be changed = always short lifetime in excited state gives rise to uncertainty in energy
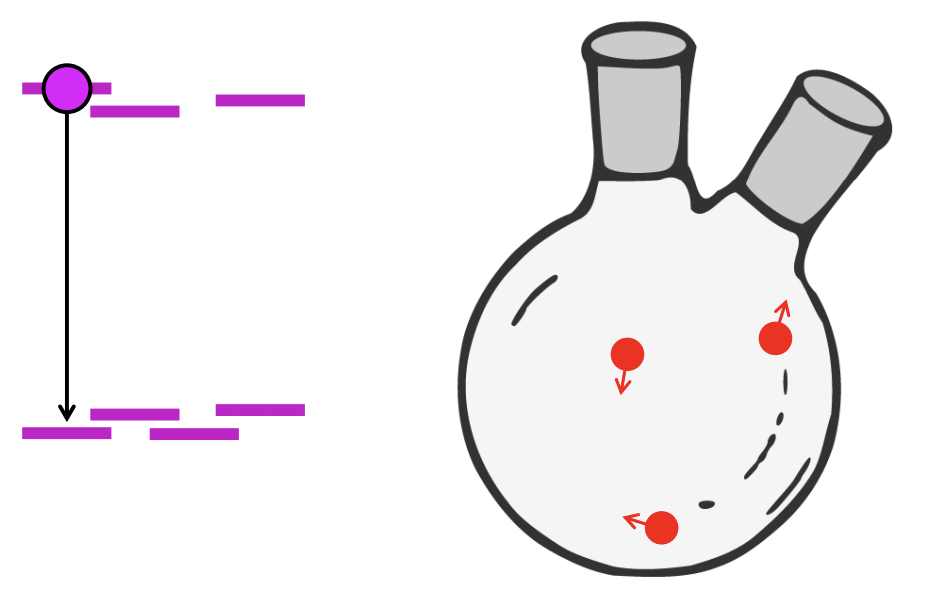
describe atomic collisions
collisions can remove energy from a state and reduce its lifetime/shift energy levels down
describe the Zeeman effect
splitting of energy levels upon application of a magnetic field interacting with m(l)
= used in NMR
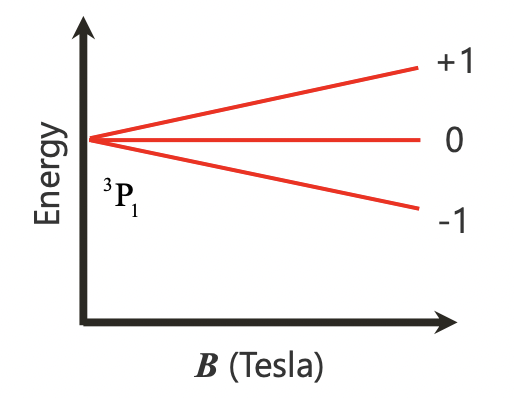
describe a magnetogram
application of the Zeeman effect
the splitting can be used to measure the strength of magnetic field in an object
= can be used in inverse to give information of magnetic field strength as expected splitting in known fields is known
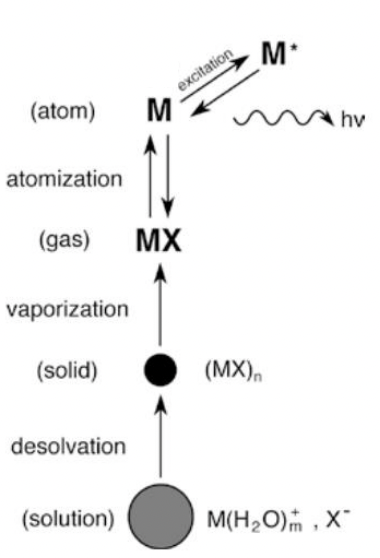
describe sample preparation for atomic absorption spectroscopy (AAS)
= used to measure elemental composition
sample/analyst is mixed into a solvent
taken up into nebuliser to produce small droplets of liquid
droplets heated to create solid particles
solid particles heated to vaporise material into gas
gas heated to atomise
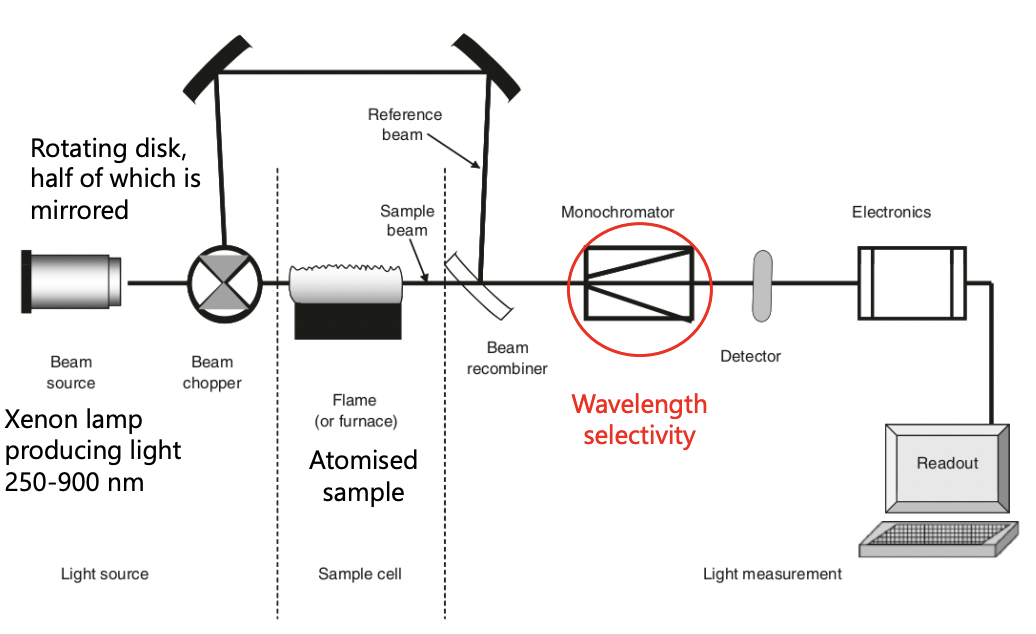
what is a good light source of AAS?
xenon lamp = large range of wavelengths (250-900nm)
describe the process of AAS (setup)
beam chopper = rotating disk changes the wavelength of light passed through
monochromator = detect single wavelength (hard to detect all wavelengths at once) = grating before improves selection (specific to atomic sample)
= reduction in intensity corresponds to the amount of light absorbed by the atoms
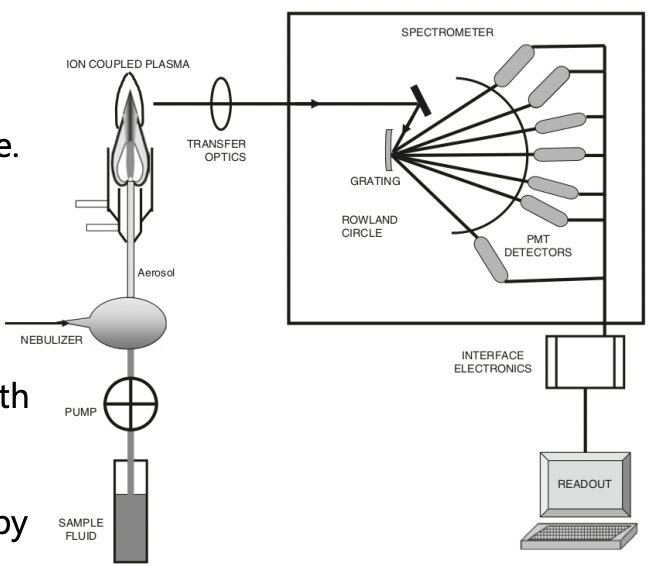
describe inductively coupled plasma atomic emission spectroscopy
= detecting emission of light from sample
ion coupled plasma = excitation
background free spectra = favourable
describe doppler broadening
in gaseous sample, atoms are moving towards/away resulting in shifting of energy levels in both directions = general broadening
moving toward detector = higher E/v observed = lower wavelength
moving away from detector = lower E/v observed = higher wavelength
describe the equation which describes doppler broadening
higher v = larger δv (frequency uncertainty)
lower v = lower δv (frequency uncertainty)
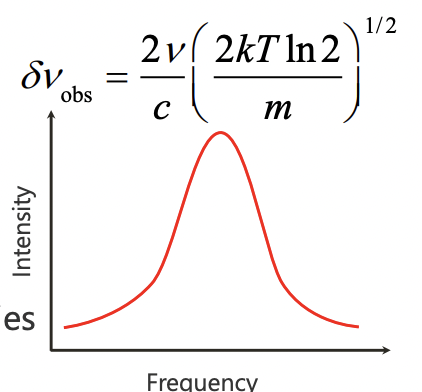
describe blue shift and red shift in context of Doppler broadening
blue shift = higher E/v = lower wavelength = moving towards observer
red shift = lower E/v = higher wavelength = moving away from observer
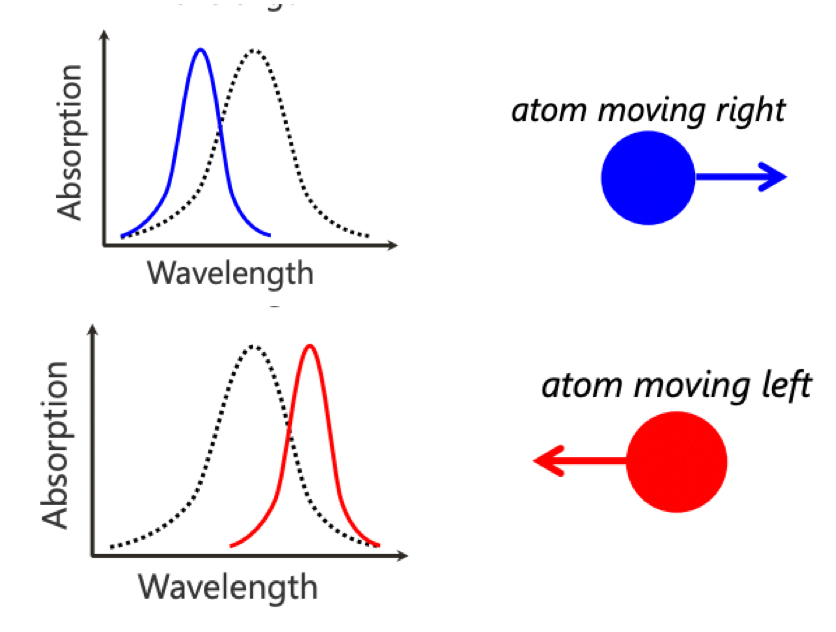
describe laser cooling
laser is tuned to a red-shifted (higher wavelength) relative to rest-frame absorption peak
atom moving towards the laser will absorb (laser wavelength is blue-shifted wrt to atom)
absorbed atoms overall momentum in direction of laser is reduced
absorbed atom in excited state will decay in a random direction. it is very unlikely this will be in the same direction as the laser
= atoms velocity towards laser has been slowed
surrounding a sample with red-shifted lasers has the effect of slowing the atomic sample
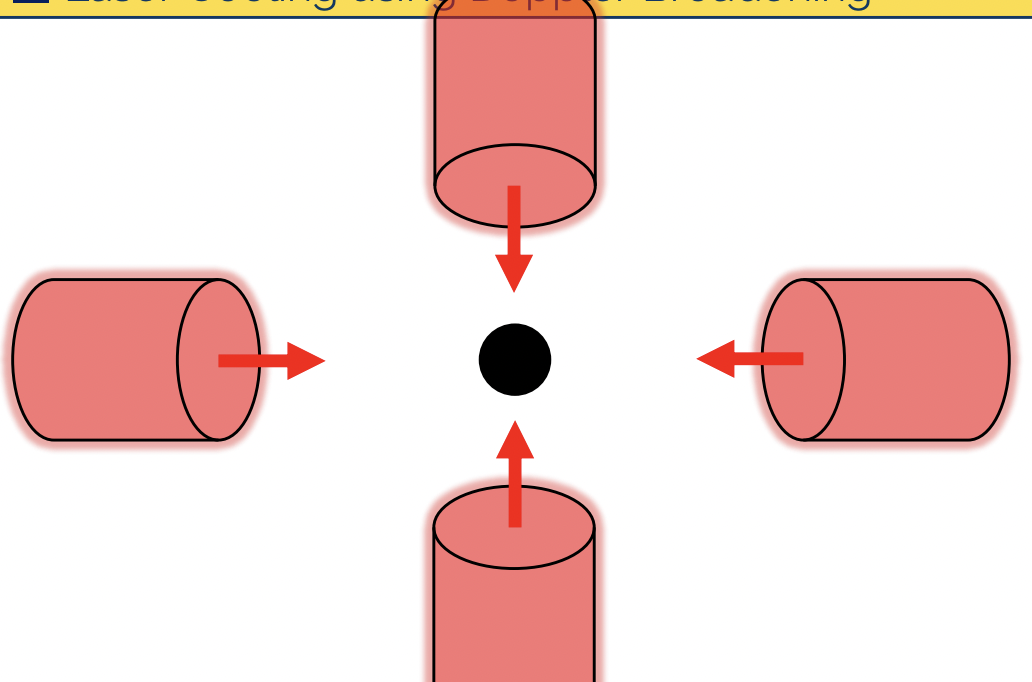
what is an application of laser cooling?
atomic clocks
GPS
describe applications of atomic spectroscopy
astronomy:
elemental composition of stars
imaging of nebulae
describe molecular spectroscopy
differs from atomic spectroscopy due to the presence of molecular orbitals
compare molecular vs atomic absorption spectra shapes
molecular absorption is broader than atomic
= different types of transitions possible
= different broadening effects:
conjugation
solvent effects
vibrations
describe optically active transitions in molecular spectroscopy
optically active = allowed
bonding → anti-bonding
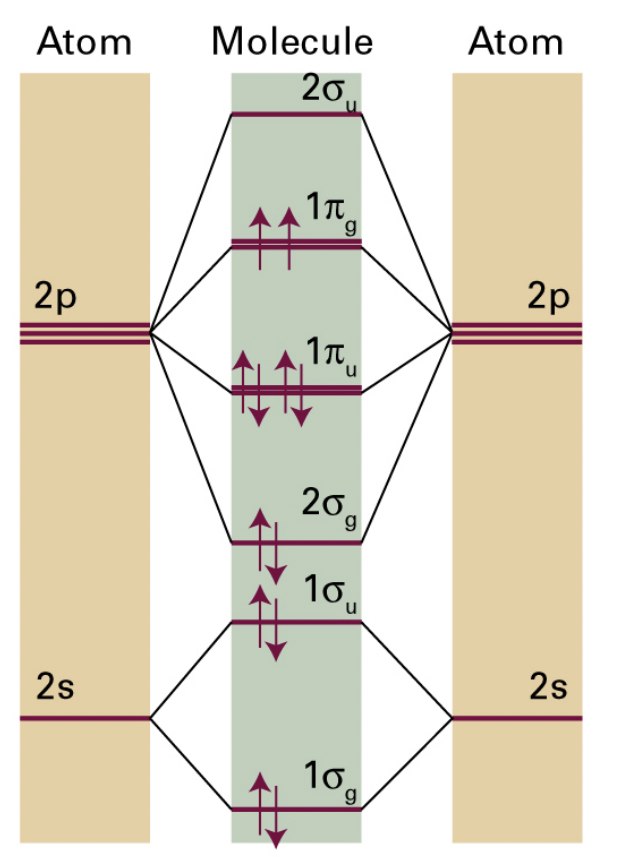
describe the effect of conjugation on molecular spectroscopy
conjugation = delocalised ∏ and ∏* orbitals join, respectively, to form single, continuous orbitals
= stabilised ∏ electrons
= decreases energy of the entire system and lowers energy of ∏*
= smaller E gap ∏→∏* = higher wavelength
conjugation ∝ wavelength of transition ∝ 1 / energy of transition, where transition is ∏ to ∏*
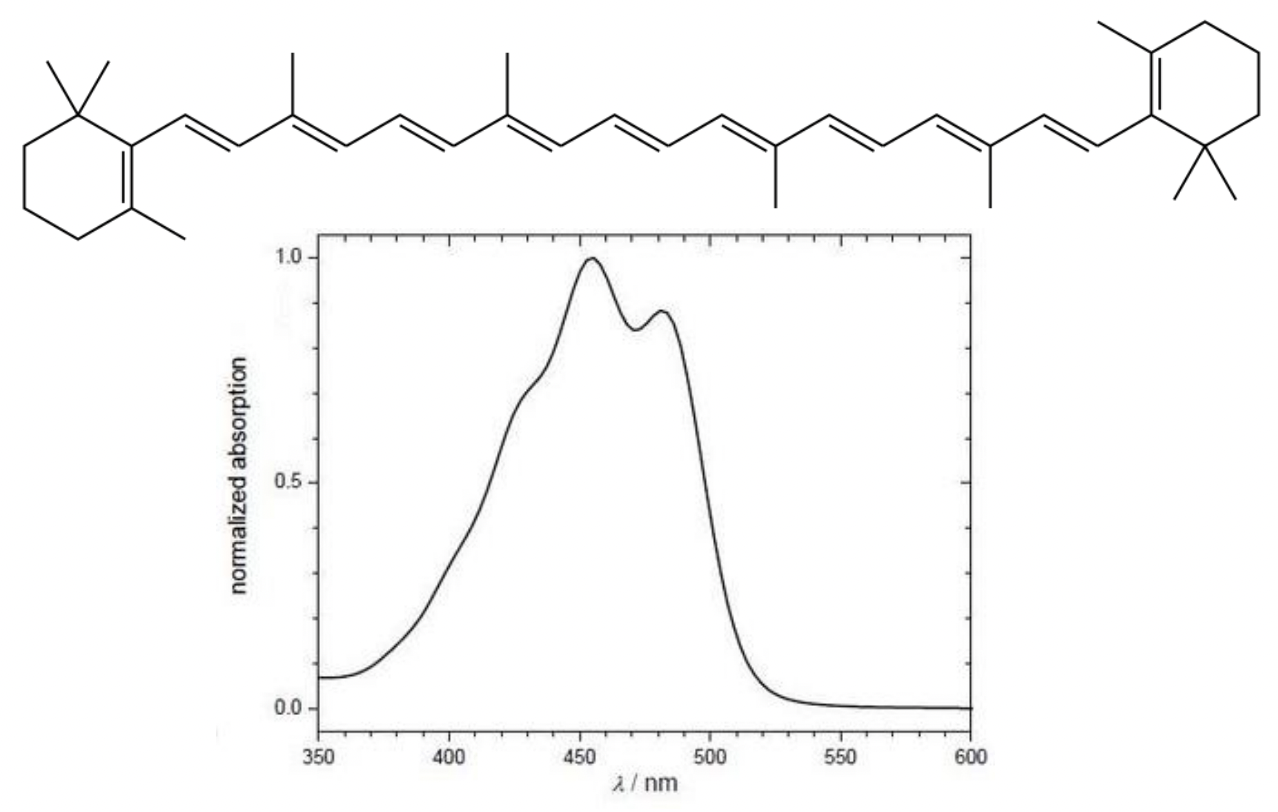
describe the colour of B-carotene
= highly conjugated molecule allows absorption in the blue/green of vis region
= orange
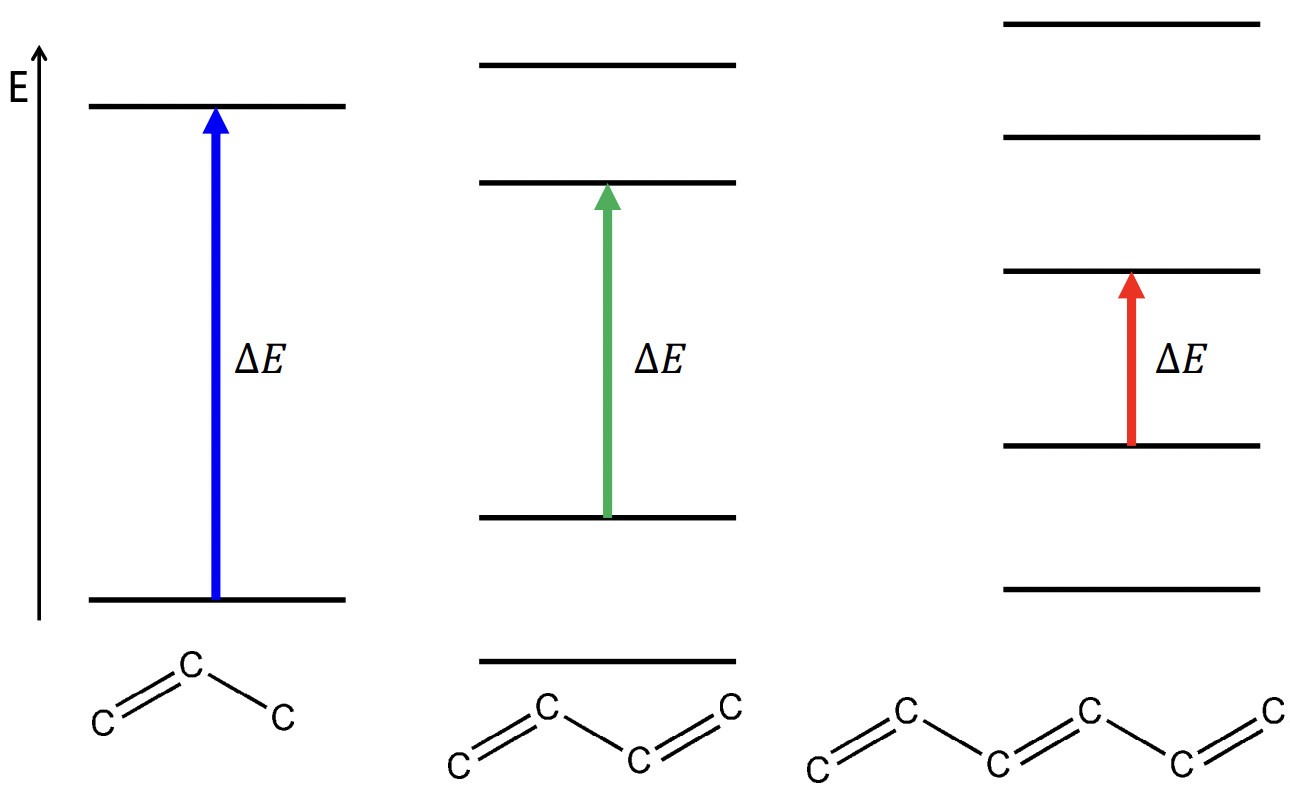
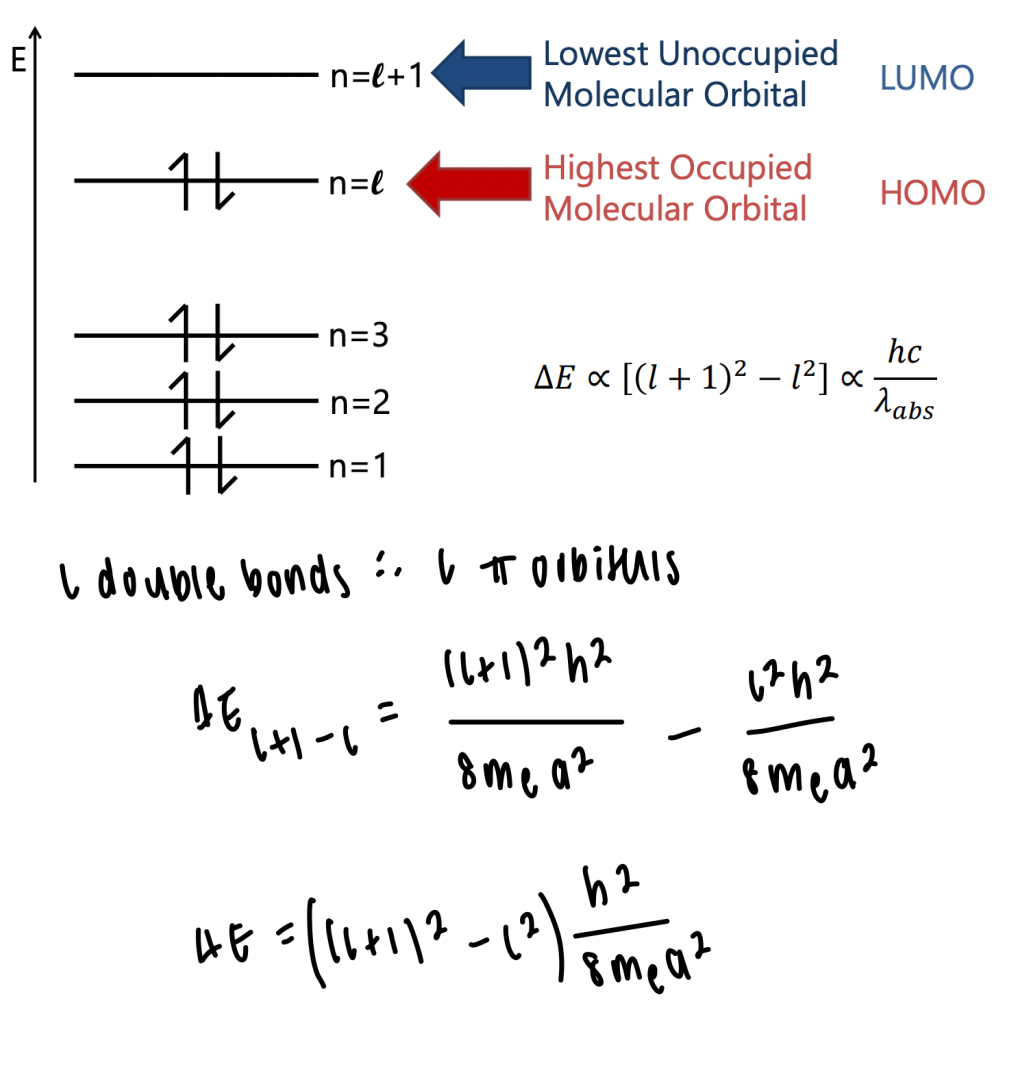
describe the application of particle in a box for linear polyenes
can be used to determine the energy gap between ∏ and ∏* orbitals
l = number of double bonds
a = length of polyene
number of electrons = 2l (since each ∏ orbital holds 2 electrons)
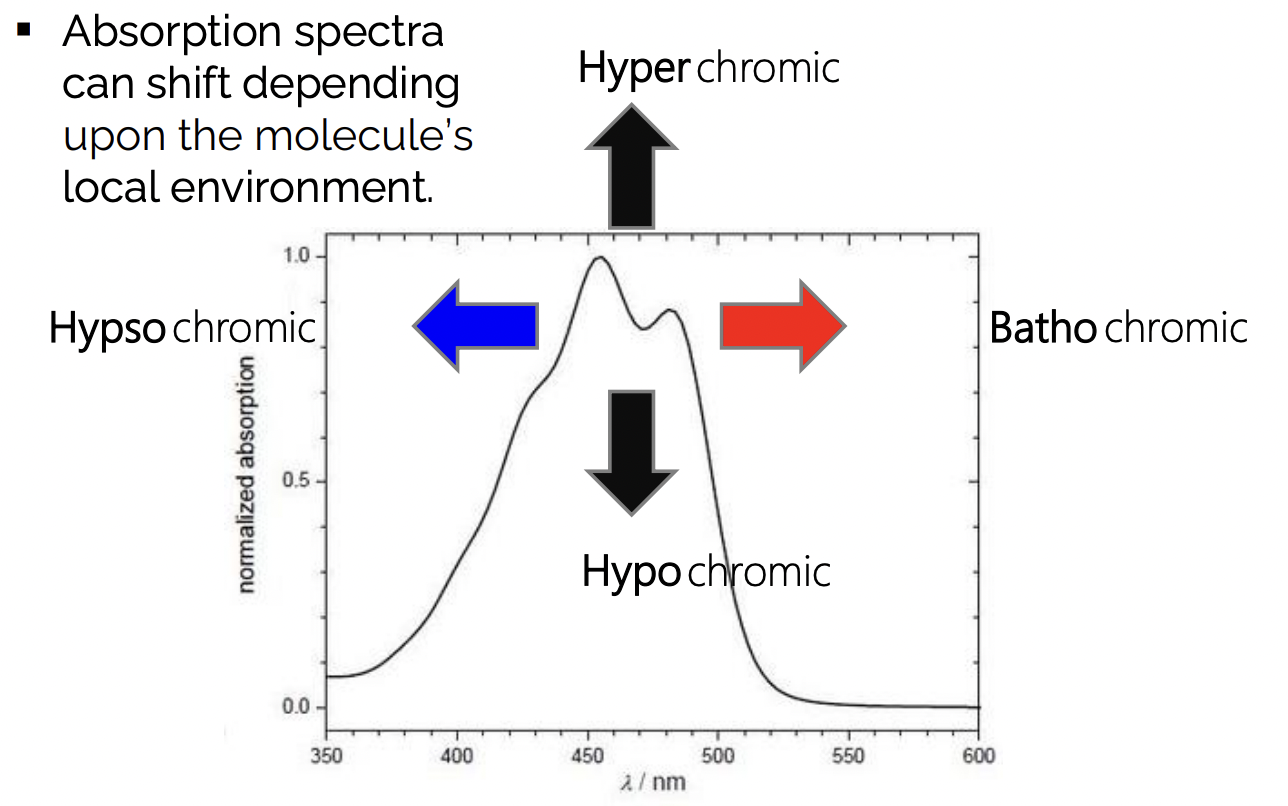
describe the different types of spectral shifts
hyperchromic = higher absorption
hypochromic = lower absorption
bathochromic = higher wavelength of absorption = ‘red-shift’
hypsochromic = lower wavelength of absorption = ‘blue-shift’
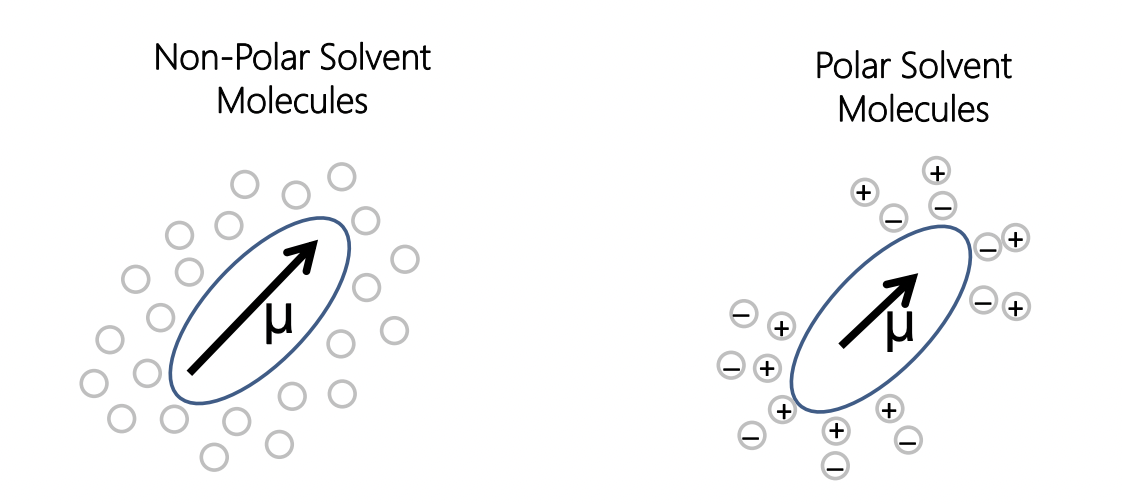
describe the effect of solvent on molecular spectroscopy
polar solvent = stabilises ground and excited state = (reduces transition dipole moment) = lower energy = bathochromic shift
solvent polarity ∝ 1/μ ∝ wavelength of transition ∝ 1/ΔE of transition
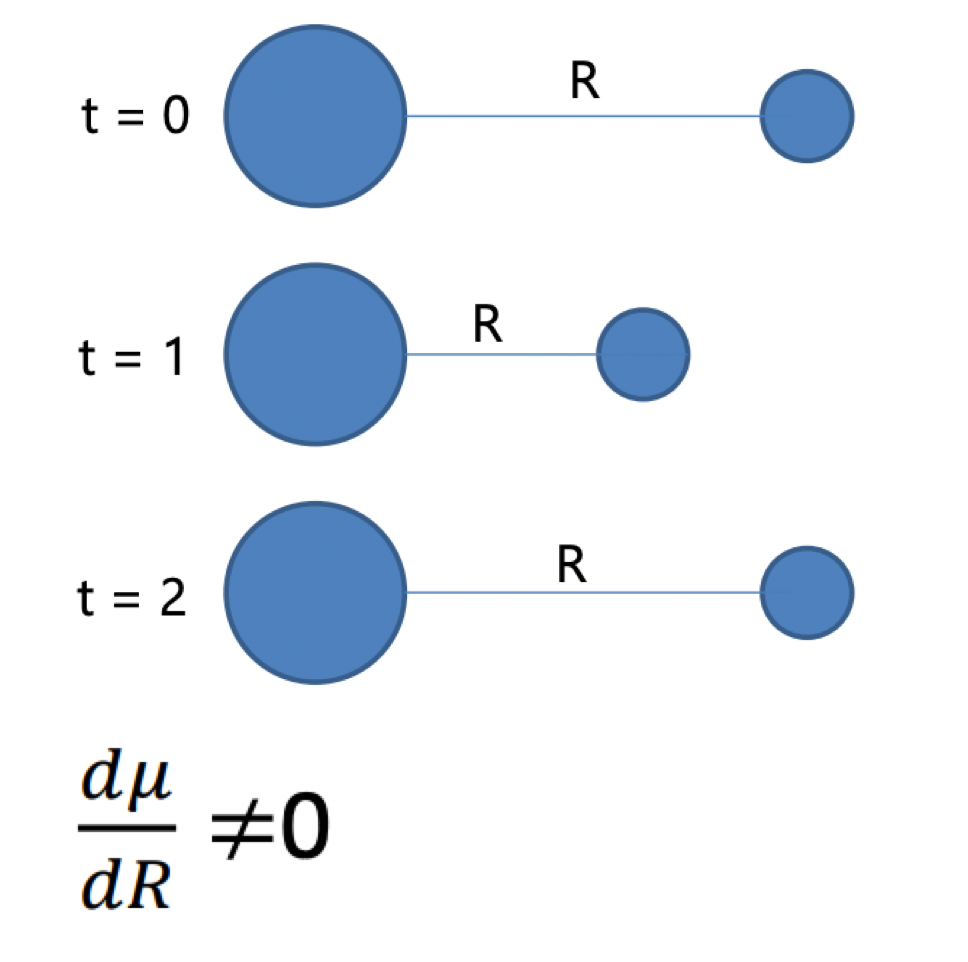
describe the effect of molecular vibrations on molecular spectroscopy
vibrations leads to variation in the dipole moment as a function of distance
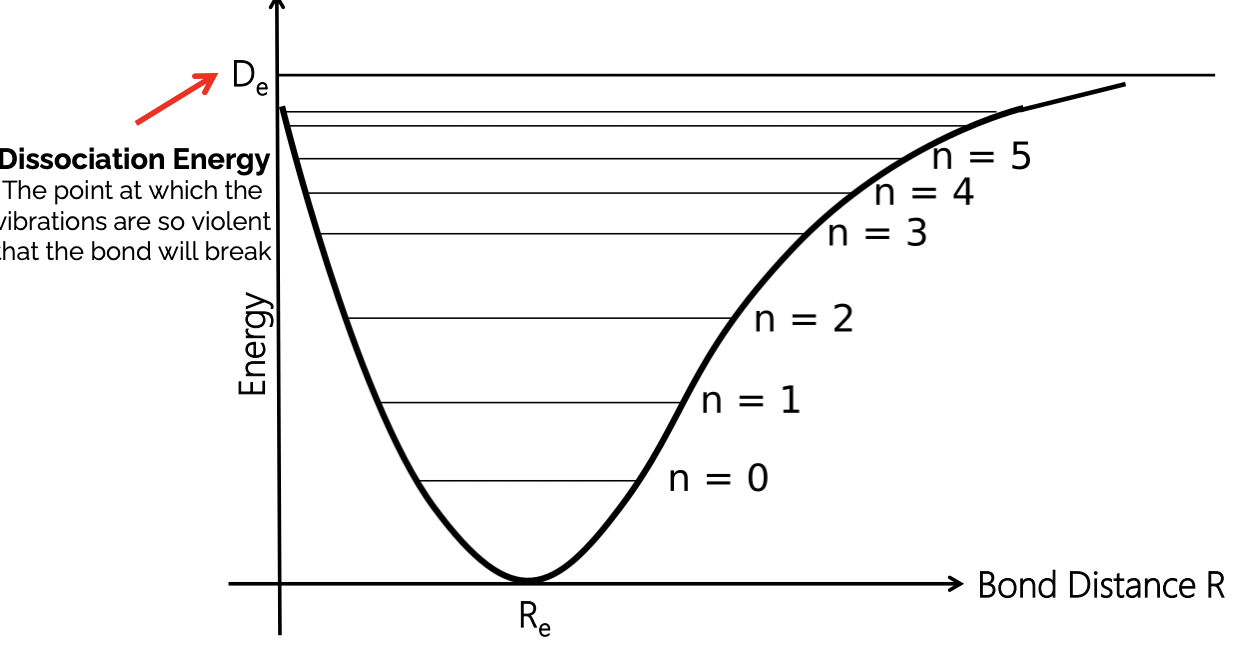
what diagram describe the vibrational sublevels
Morse potential
n = vibrational quantum number ~ extent of vibrations
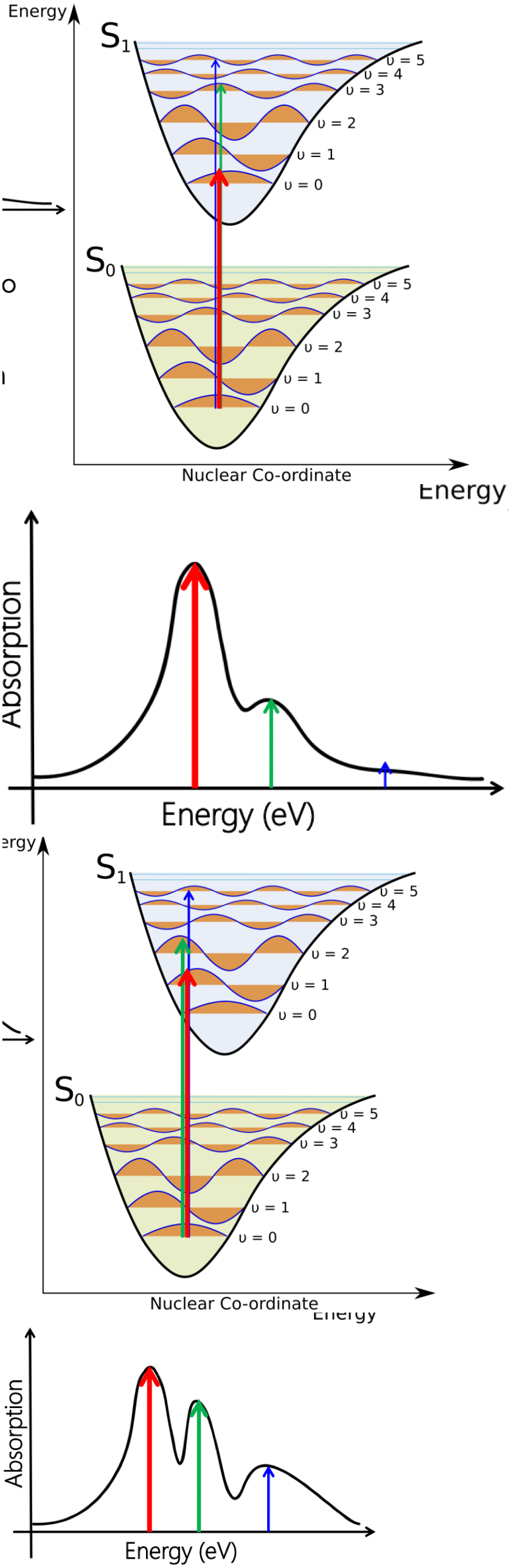
describe vibrational fine structure of an energy level diagram
each energy levels (ground and excited) has a vibrational sub structure, as described by the Morse potential
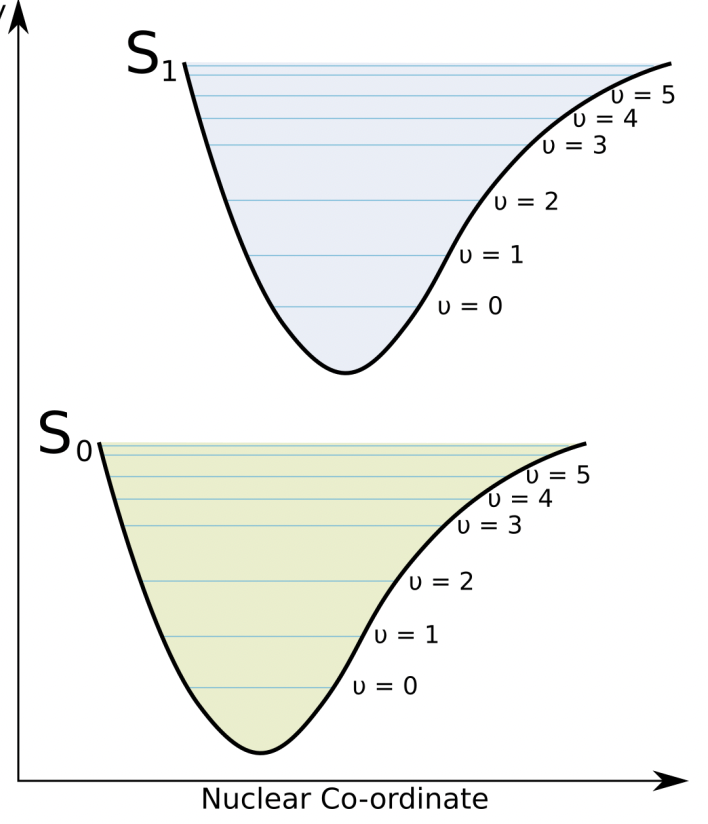
!! why is the excited state nuclear coordinate shifted w.r.t. ground state
larger inter-nuclear separation caused by change in electron distribution
!! what is the Franck-Condon principle
electronic transition probability is proportional to the wavefunction overlap of ground and excited state potentials
largest vibrational wavefunction overlap = strongest transition
(no effect on wavelength/colour of absorption)
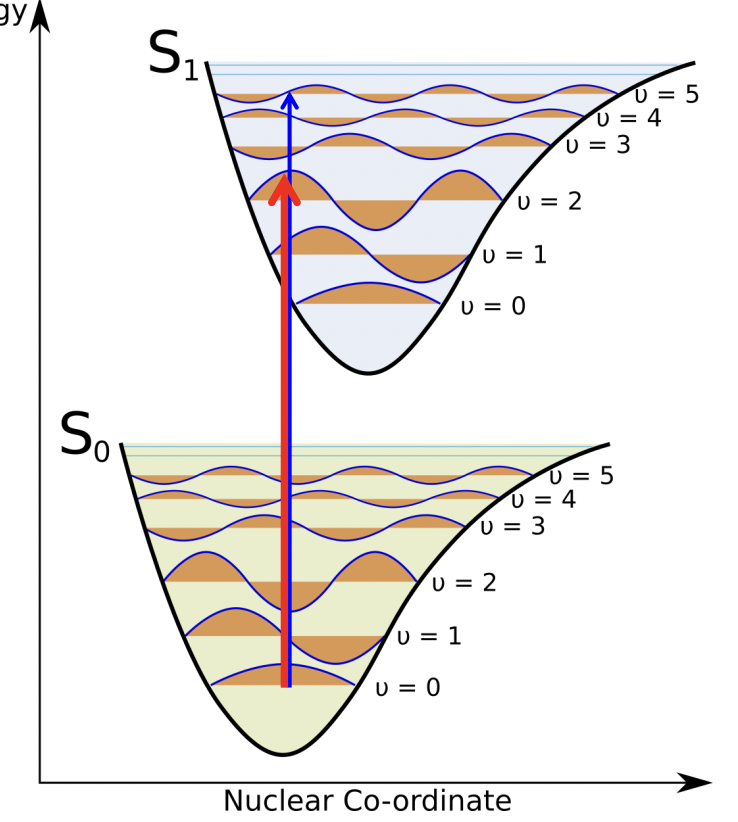
!! what is the Born-Oppenheimer Approximation
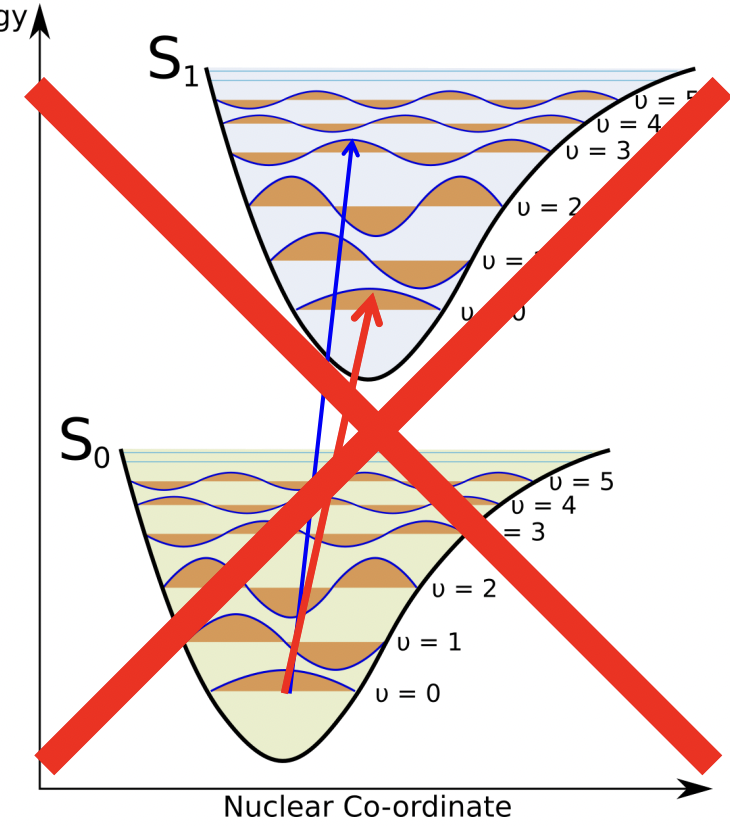
electronic transitions are much faster than vibrations. hence, electronic transitions are strictly vertical and the vibrational states are unchanged.
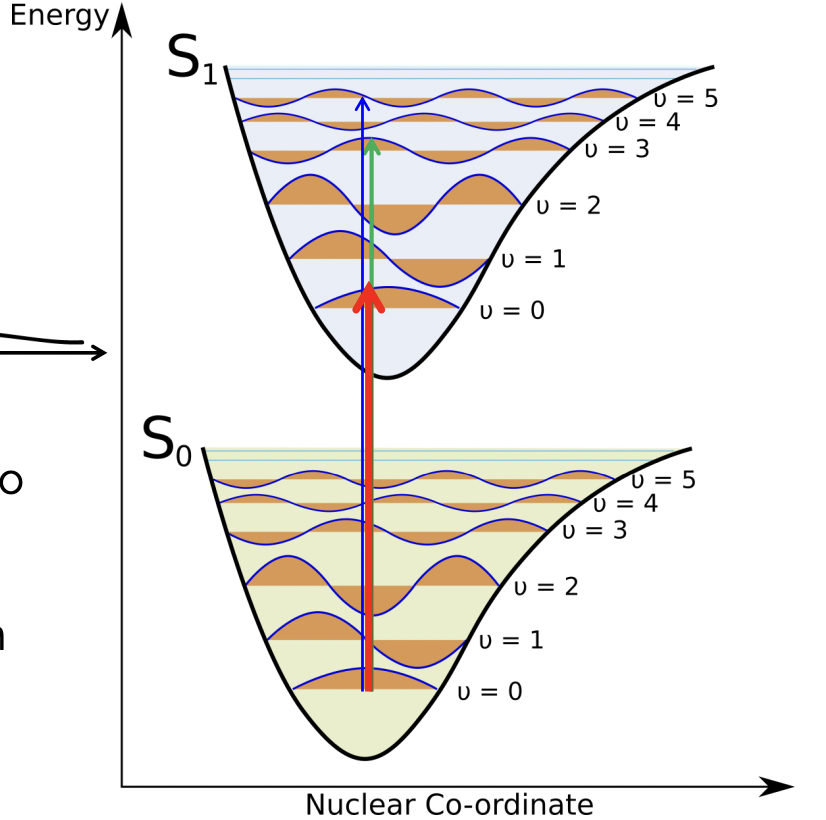
describe the spectra for this electronic transition
3 peaks all from one electronic transition
red = most intense
blue = least intense
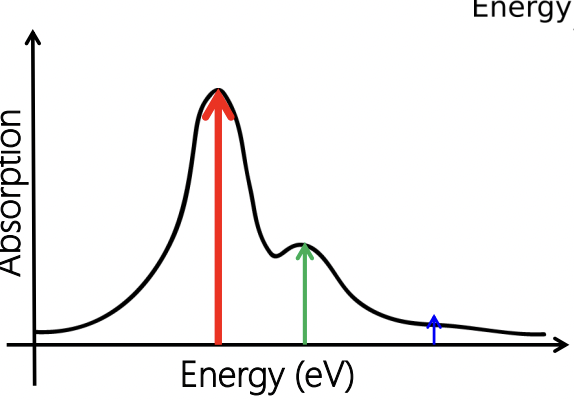
describe the extent of nuclear co-ordinate offset with intensity ratio of peaks
small offset = less well distributed peaks
large offset = more well distributed peaks
describe the path of excitation/relaxation
excitation → higher excited vibrational state = ‘hot’ excited state
fast non-radiative decay of hot modes to bottom of S1
radiative decay to ground state, emitting a photon
relaxation → lower ground vibrational state
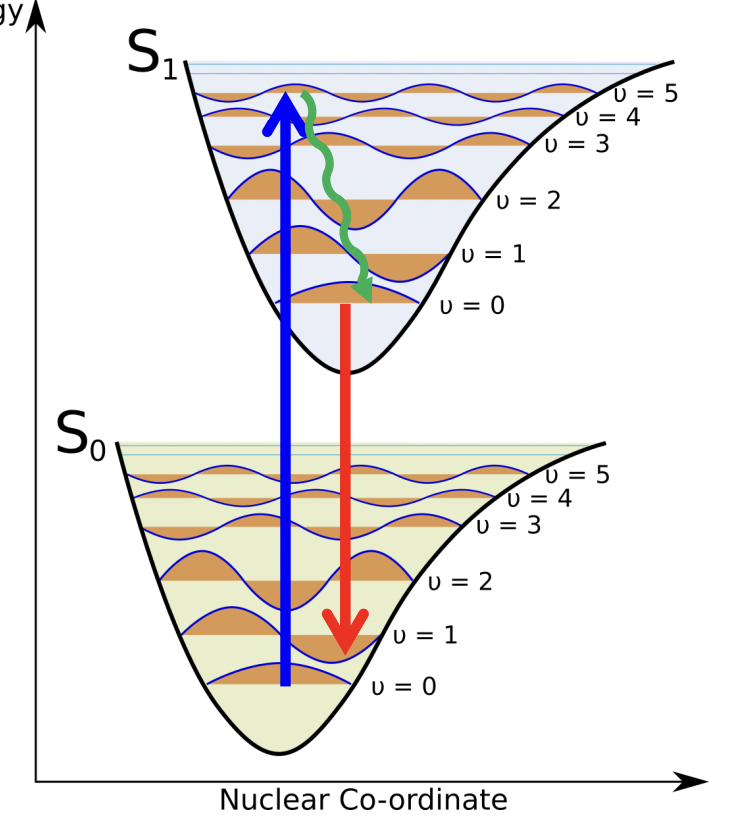
describe photoluminescence
if the excited state was created by optical absorption:
relaxation = photoluminescence