Lecture 5 Polymer Degradation and Design of Degradable Biomaterials
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
Degradation and Erosion
Degradation: ____ process resulting in the ____
of covalent bonds in the ___ or ____._____
______
_____
Erosion: ____ change in size, shape or mass as a
result of either ____ or simply ___Bioerosion
Water-____ being converted into water-___ under physiological condition. Including both physical (___) and chemical (____ __ _____) process.
Degradation: chemical process resulting in the cleavage
of covalent bonds in the backbone or crosslinks.Oxidative
Enzymatic
Hydrolytic
Erosion: physical change in size, shape or mass as a
result of either degradation or simply dissolutionBioerosion
Water-insoluble being converted into water-soluble under physiological condition. Including both physical (dissolution) and chemical (cleavage of backbone) process.
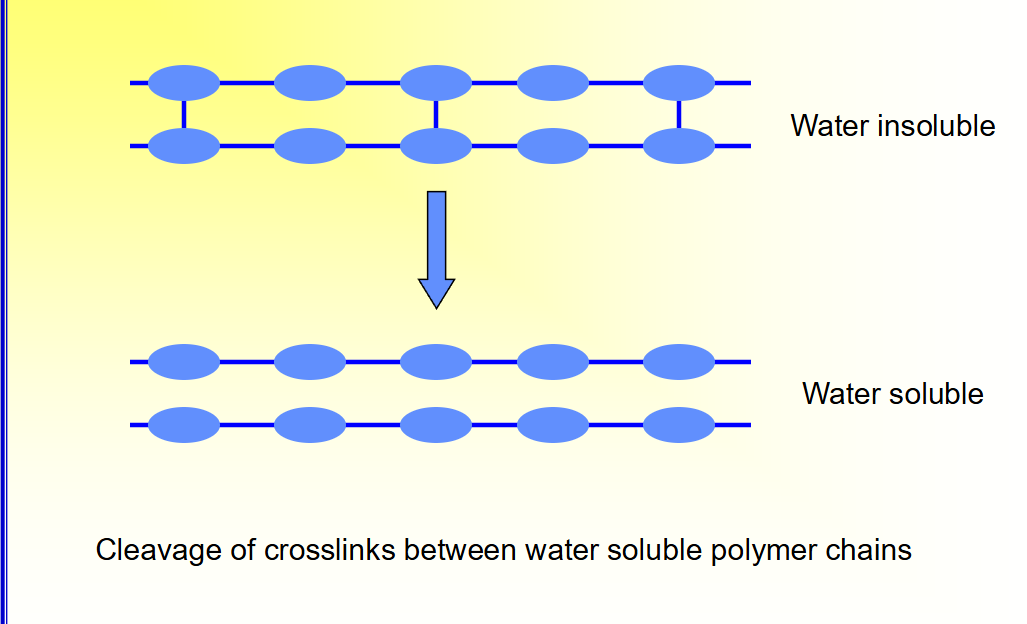
How does erosion driven by chemical processes look 1?
Cleavage of crosslinks between water soluble polymer chains
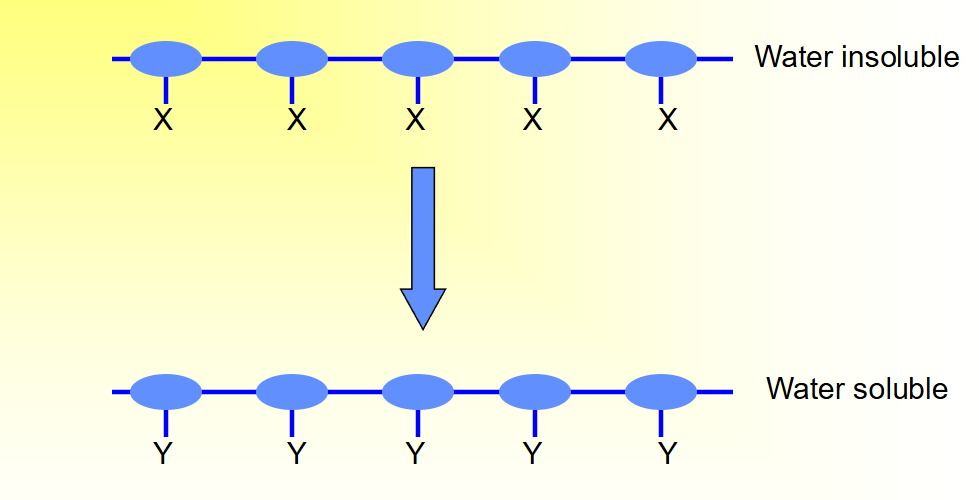
How does erosion driven by chemical processes look 2?
Transformation or cleavage of side chains (X) leading to
the formation of polar or charged groups (Y).
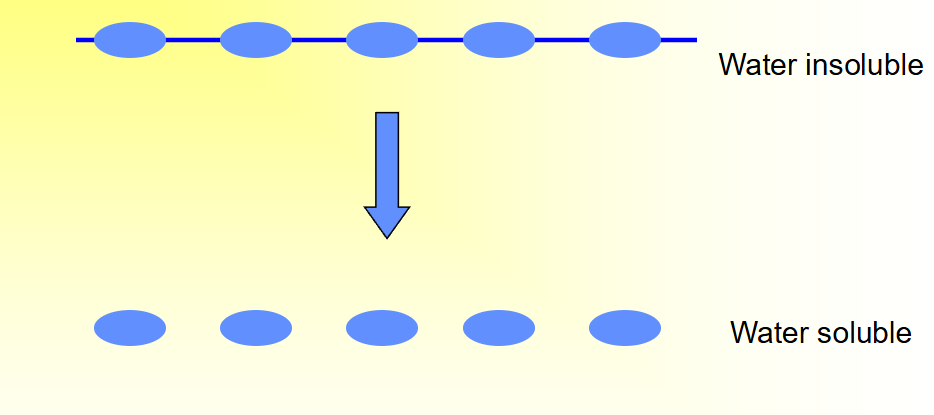
How does erosion driven by chemical processes look 3?
Cleavage of backbone linkages between polymer repeat units
What are some physical modes of Bioerosion?
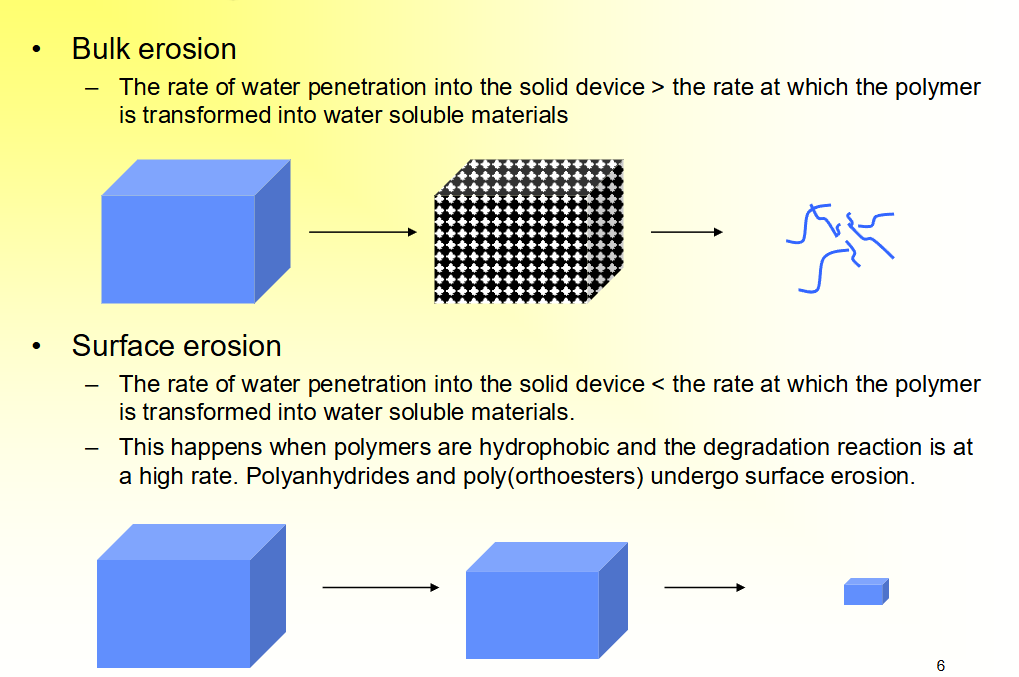
Bulk erosion
The rate of water penetration into the solid device > the rate at which the polymer
is transformed into water soluble materials
Surface erosion
The rate of water penetration into the solid device < the rate at which the polymer is transformed into water soluble materials.
This happens when polymers are hydrophobic and the degradation reaction is at a high rate. Polyanhydrides and poly(orthoesters) undergo surface erosion.
Polymer Degradation-Oxidative
Polymer chain attacked by reactive species (free radicals or oxidative ions produced by the inflammatory process or metal implant corrosion).
Initiation steps:
Homolysis
• R-R ➔2R.Heterolysis
• R-R➔R++R-
Not a common choice for designing a degradable polymer
Exception: ROS (reactive oxygen species) responsive materials
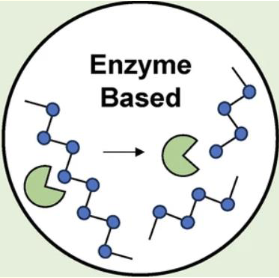
Polymer Degradation - Enzymatic
Major degradation mechanism for natural polymers
Selective: protease, esterase, lipases, glycosidase, nucleosidase
Not predictable as the levels of enzymes varies from time to time, location to location and subject to subject
Polymer may be designed to degrade by a specific cell type or specific biological condition
Surface erosion can be realized by enzyme-mediated degradation
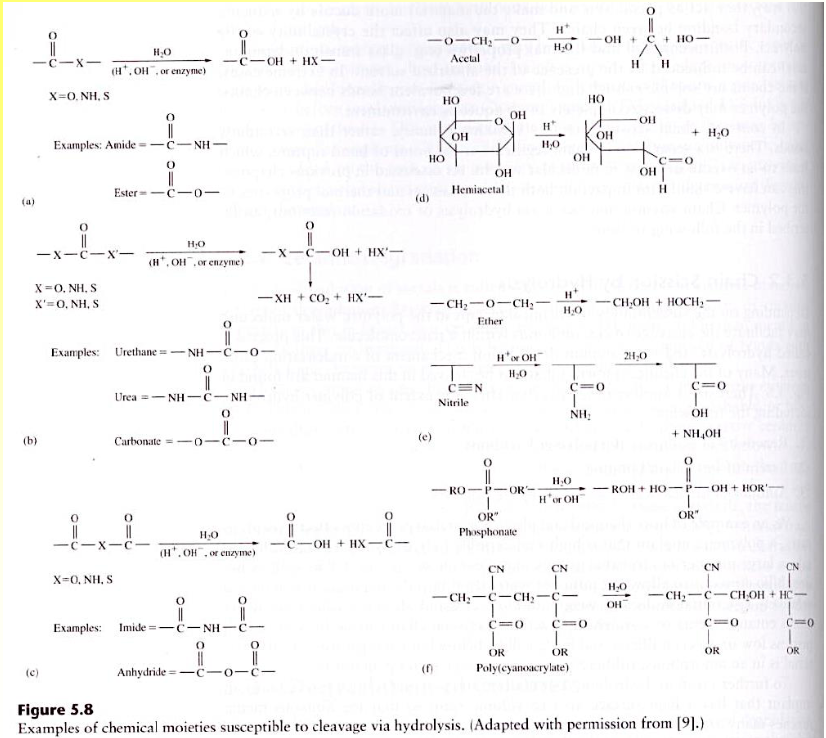
Polymer Degradation-Hydrolytic
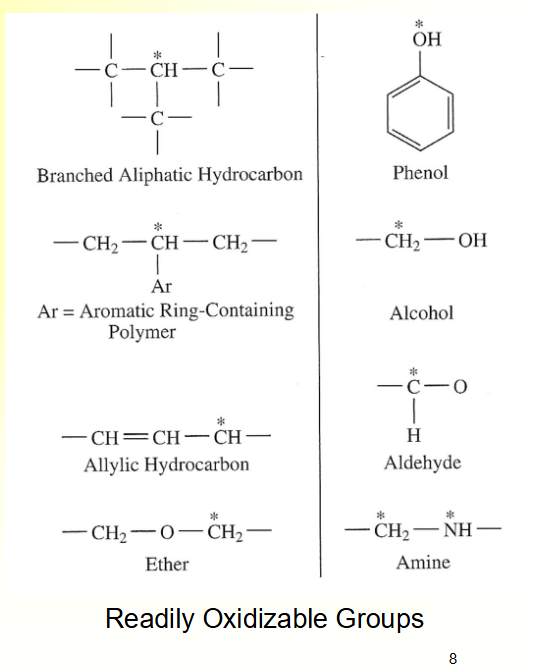
Hydrolyzable groups: carbonyl bonded to heterochain elements (O, N, S), full list see Figure 5.8 next slide
Host induced
– Neutral water
– Ion-catalyzed
– pH-catalyzed, low pH occurs at the acute inflammation or infection site
– Enzyme-catalyzed
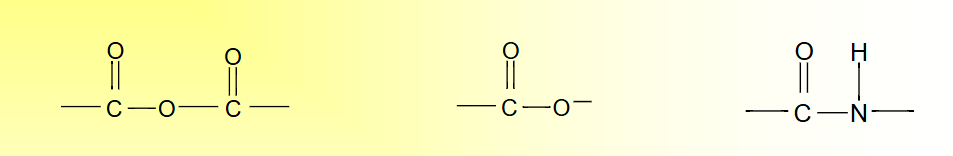
Factors Influencing the Hydrolytic Erosion
• Chemical stability of the linkage
Rate of degradation:
polyanhydrides > polyesters > polyamide
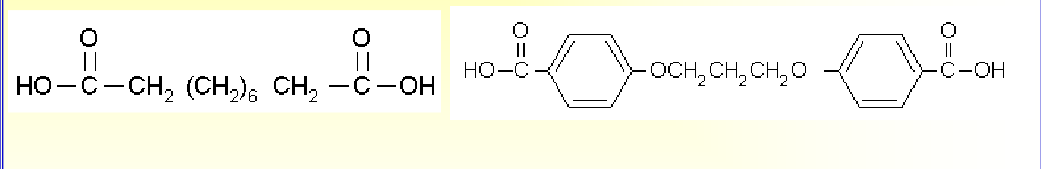
Factors Influencing the Hydrolytic Erosion II
The erosion rate is strongly dependent on the ability of
water to penetrate into the polymeric matrix.Hydrophobicity of the monomer
– More polar groups, -OH, -COOH, -C=O, less hydrophobic
– More nonpolar groups, phenyl, -CH3, more hydrophobic
Polyanhydride made of hydrophilic sebacic acid erodes 3
orders of magnitude faster than that made of hydrophobic
bis(carboxy phenoxy)propane
More factors influecing the hydrolytic erosion:
Microstructure, ___ vs. ___ (glassy vs. rubbery)
____ structure is resistant to the ___ penetration. Semicrystalline PLLA vs. amorphous D,L-PLA
_____ materials degrade faster when they are above Tg (____ state) than below Tg (____ state)
____ molecular weight (molecular weight ____)
____ the initial molecular weight, quicker to break down
Geometry and morphology, more ____ area, faster erosion
Additives, catalysts, plasticizers, may ___ or ___ H2O penetration
Mechanical stress: points of ____ ___ ____ degrade more rapidly;
Processing and fabrication, ~morphology, microstructure and residual stress
Microstructure, crystalline vs. amorphous (glassy vs. rubbery)
Crystalline structure is resistant to the water penetration. Semicrystalline PLLA vs. amorphous D,L-PLA
Amorphous materials degrade faster when they are above Tg (rubbery state) than below Tg (glassy state)
Initial molecular weight (molecular weight distribution)
Lower the initial molecular weight, quicker to break down
Geometry and morphology, more surface area, faster erosion
Additives, catalysts, plasticizers, may inhibit or aid H2O penetration
Mechanical stress: points of residue stress concentration degrade more rapidly;
Processing and fabrication, ~morphology, microstructure and residual stress
Degradable Biomaterials
Advantages:
Permanent implants may cause chronic inflammation, stress shielding, etc.
Degradable materials are gradually absorbed by the body and don’t permanently
leave traces of residuals in the implantation sites.The degradation rate can be adjustable according to the application needs
Biocompatibility considerations:
Neither the polymer nor its degradation products nor the subsequent metabolites
should provoke excessive inflammation or toxicity
Storage, packaging, and sterilization stability:
Avoid moisture
Packed in air-tight aluminum-backed plastic foil, and stored at low temperature
Sterilization
Autoclaving and irradiation, bad
Ethylene oxide, OK
Applications of degradable biomaterials
Drug Delivery
Degradable polymer serves as a matrix or encapsulation.
The degradation properties are tailored to achieve optimal release kinetics of the drug or active agent
Temporary Support: sutures, bone fixation devices
Provide temporary mechanical support until the nature tissue heals and regains its strength
Adjust the degradation rate to the healing process
Tissue Engineering Scaffold
The surface should permit cell adhesion and growth;
The scaffold degradation rate should match the rate of tissue regeneration by the cell type of interest
Multifunctional
Example 1: Degradable bone nails or bone screws loaded with drug
Example 2: Biodegradable and drug-eluting stents

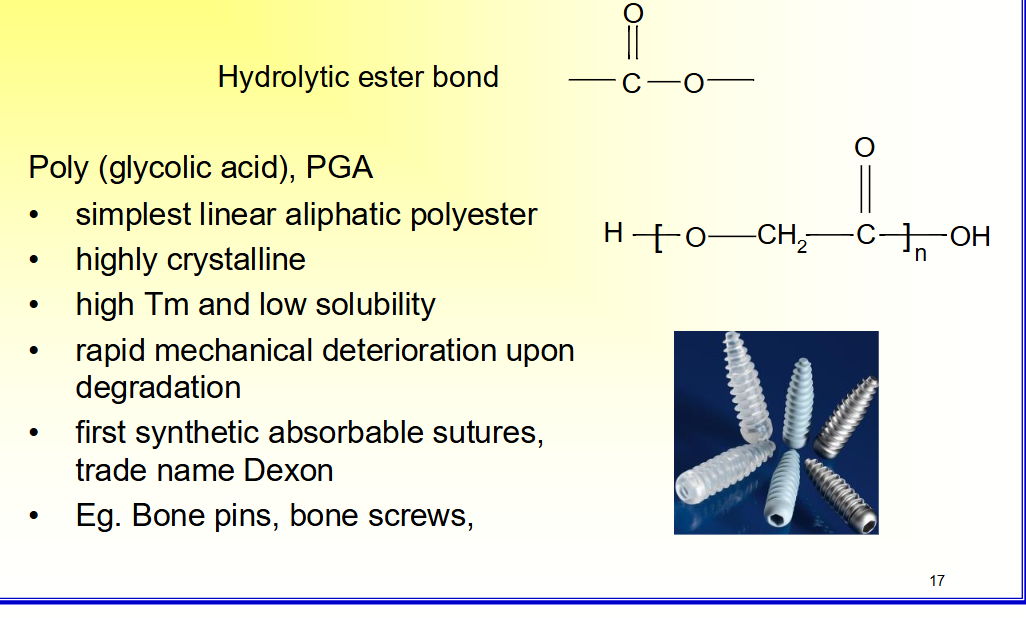
What are Polyester
Type of degradable polymer
Poly (glycolic acid), PGA
• simplest linear aliphatic polyester
• highly crystalline
• high Tm and low solubility
• rapid mechanical deterioration upon
degradation
• first synthetic absorbable sutures,
trade name Dexon
• Eg. Bone pins, bone screws
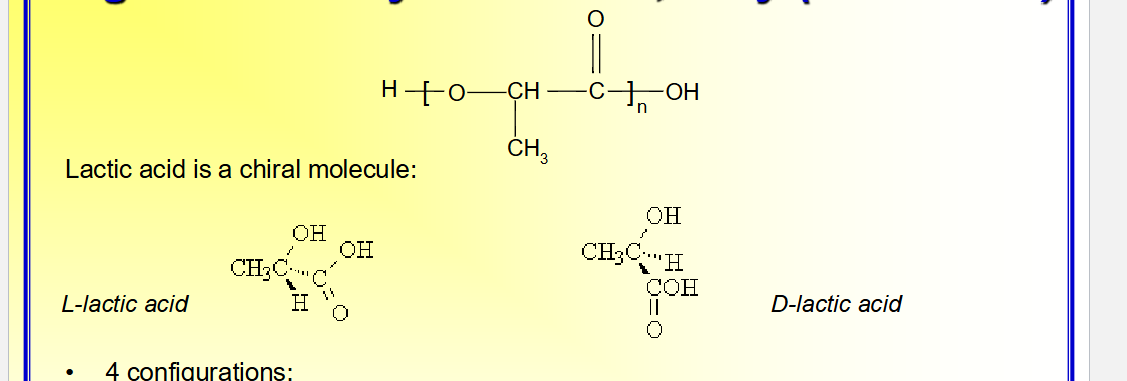
Degradable Polyester: PLA, Poly (lactic acid)
4 configurations:
stereoregular D-PLA (PDLA) and L-PLA (PLLA). Usually PLLA is used because the hydrolysis product L-lactic acid is the naturally occurring lactic acid
racemic D,L-PLA
meso-PLA obtained from D,L lactide
Semi-crystalline PDLA and PLLA; amorphous D,L-PLA
amorphous D,L-PLA is used in drug delivery where monophasic matrix is important;
semi-crystalline PLLA is good for applications requiring high mechanical strength and
toughness, such as sutures and orthopedic devices
Copolymer of LA and GA (PLGA)
PLLA is more hydrophobic than PGA, limits the
water uptake and reduce the backbone hydrolysisAddition of PLA component to PGA helps to reduce the hydrolysis rate
GA:LA 90:10 is used as suture materials (Vicryl and Polyglactin 910)
There is no simple linear relationship between the ratio of GA:LA and the mechanical and degradation properties of the corresponding copolymers (see homework 2)


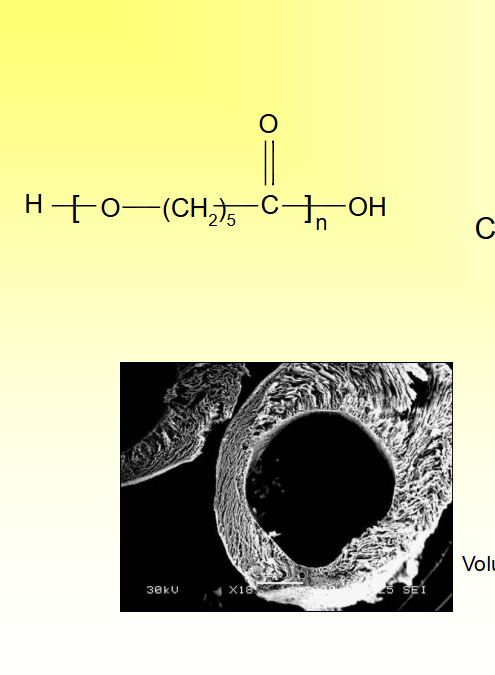
Poly (-caprolactone)
Sutures and drug release
Slower degradation than PLA
Semi-crystalline
Copolymers have been made with PGA
Polyanhydride
• Among the most reactive and hydrolytically unstable polymers used as biomaterials
• Fast degradation, excellent in vivo biocompatibility
• React with drugs having free amino groups or other nucleophilic
functionalities → limit the types of drugs that can be successfully
incorporated.
• Predominantly used in drug delivery prepared by compression molding or
microencapsulation.
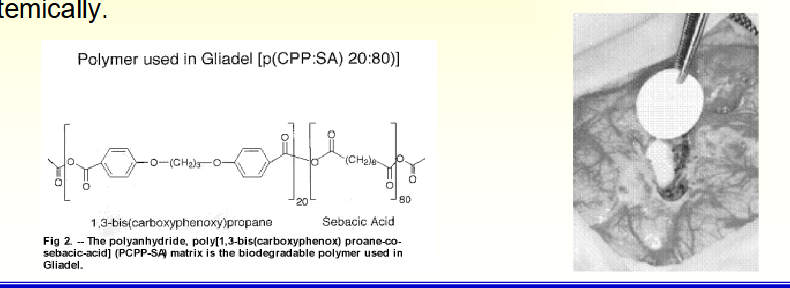
• Gliadel Wafer: used for intracranial delivery of bis-chloroethylnitrosourea (BCNU) for brain cancer. The wafers are implanted directly into the brain to deliver BCNU that has deleterious side effects when administered
systemically.