Aldehydes and Ketones
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
difference between aldehydes and ketones?
aldehydes carbonyl group is always at the end,
ketones are in the middle
what is a carbonyl group?
C=O, carbon double bonded to an oxygen
what are aldehydes reduced into?
primary alcohols
what can aldehydes be further oxidised into?
carboxylic acids
what are ketones reduced into?
secondary alcohols
why cant ketones be reduced further?
they do not have a hydrogen attached to the carbonyl group
what are primary alchols oxidised into?
aldehydes
what are secondary alcohols oxidised into?
ketones
reduction definition in organic chemistry?
when a carbon forms a bond with a less electronegative element, usually hydrogen
oxidation definition in organic chemistry?
when a carbon forms a bond with a more electronegative element, like oxygen
what reducing agent is used to reduce aldehydes and ketones?
NaBH4 (aq), sodium borohydride
LiAlH4 (aq), lithium aluminium hydride
what are both of these agents a source of?
hydride ions
before reduction, how are hydride ions formed from the reducing agents?
reducing agent is dissociated when dissolved in water:
reducing agent is dissociated when dissolved in water:
NaBH4 -> Na+ + BH4-
BH4- : in a reaction one B-H bond breaks forming a nucleophile H:- as homolytic fission occurs
aldehyde reuduction mechanism?
1 hydride forms a bond with carbon
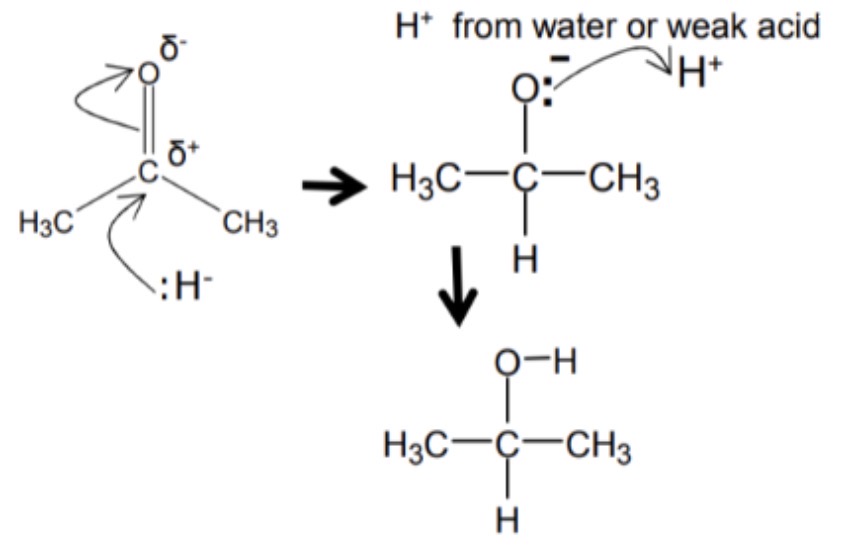
ketone reduction mechanism?
nucleophilic additon:
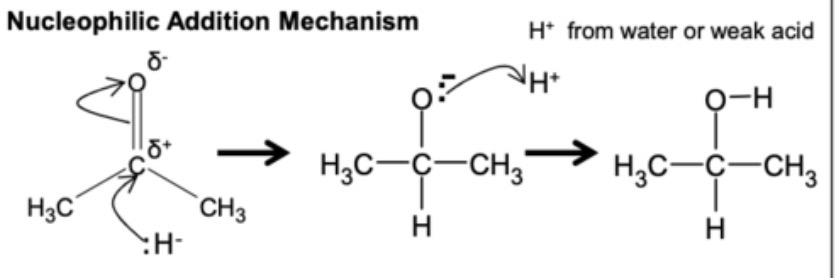
what type of mechanism is reduction of ketones and aldehydes?
nucleophilic addition
what is nucleophilic addition?
reaction where the nucleophile is added to the electrophilic carbon atom, typically attached to a carbonyl group
difference between nucleophilic addition and nucleophilic substitution?
addition is where the nucleophile is added, substitution is where the nucleophile replaces the leaving group attached to the electrophilic C atom
why do ketones and aldehydes have similar reaction mechanisms?
they both have a polar carbonyl group
(+)C=O (-)
equation for reduction of aldehydes?
aldehyde + 2[H] -> primary alcohol
structural formula used
equation for reduction of ketones?
ketone + 2[H] -> secondary alcohol
what does the 2[H] represent in the equations for the reduction of ketones and aldehydes?
simplified to represent hydrogens from the reducing agent (NaBH4) and water (H20)
when an unsymmetrical ketone is reduced, what isomer is produced?
optical isomer (as a chiral carbon is produced)
what is an unsymmetrical ketone?
type of ketone where the two groups attached to the carbonyl carbon are different., meaning that the molecule does not have a plane of symmetry through the carbonyl group
why can we not test to see which optical isomer is produced in the reduction of unsymmetrical ketones?
a racemic mixture is produced
how is this racemic mixture produced?
1 within carbonyl groups in aldehydes and ketones, the carbons bonds form a triagnoal planar arrangement
2 when reacting with a reducing agent, a bond between the carbon and hydride can form either side,
3 the chances are 50/50
4 so when the reaction takes place, a 50/50 mix of enantiomers is produced = RACEMIC MIXTURE
difference between KCN and NaCN to HCN?
KCN and NaCN are solid ionic salts,
HCN is a covalently bonded gas (very dangerous)
what does nucleophilic addition of aldehydes/ketones with a cyanide ion produce?
hydroxyitriles (under certain conditions)
what is a hydroxynitrile?
molecule with both an alchol and nitrile group (OH & CN)
does CN have a single, double or triple bond?
triple
conditions required for aldehydes/ketones to produce hydroxynitriles from nucleophilic addition?
source of CN ions (aq)
dilute sulphuric acid
hazards with carrying out nucleophilic addition with KCN in a lab?
KCN is deadly when ingested,
KCN can react to produce the deadly gas hydrogen cyanide
nucleophilic addition with aldehydes and cyanide?
1 C-C bond forms from delta positive carbon and negative carbon (-:CN)
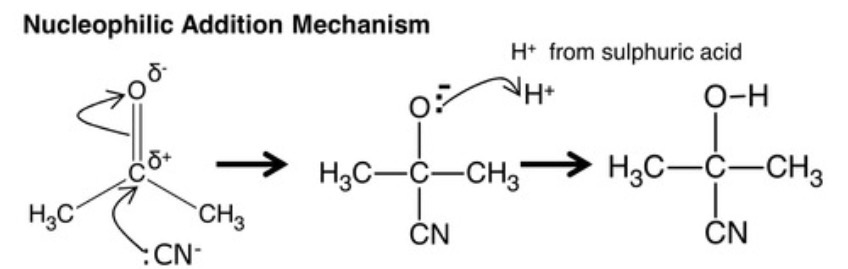
where is the H+ ion from that bonds with the negative oyxgen?
acidic sulfuric acid (H2SO4)
nucleophilic addition mechanism for ketones with cyanide?
1 formation of a C-C bond (-:CN + C)
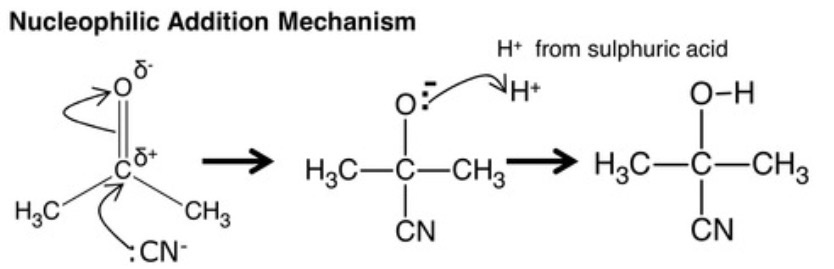
name all the nucleophilic addition reactions?
production of hydroxynitriles
reduction of ketones and aldehydes
difference between naming hydroxynitriles and naming other organic molecules?
we always count the carbon in the nitrile as position number 1 in the carbon chain, where with others we find the longest carbon chain
prefix and suffix for naming hydroxynitriles?
-nitrile
hydroxy-
e.g. 2-ethyl-2-hydroxylpentanenitrile
how to write equations for the formation of hydroxynitriles?
structural formula of alchol/ketone
sulfuric acid and KCN as other reactants
structural formula for hydroxynitrile
KHSO4 as the other product
what is the other product that is always produced with the nucleophilic addition of ketones/aldehydes with KCN?
potassium bisulfate, KHSO4
what reactions produce chiral carbons and therefore optical isomers?
nucleophilic addition with unsymmetrical ketones
nucleophilic addition with aldehyde (hydroxynitriles)
reduction of unsymmetrical ketones
will hydroxynitriles produced in the nucleophilic addition of aldehydes rotate plane polarised light?
no!!!!!! , a racemic mixture is produced
how is this racemix mixture is produced?
the chances of forming C-CN bond is 50/50, so products are non-superimposable, mixture is then optically inactive and wont rotate plane polarised light