describe chemical reactions
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
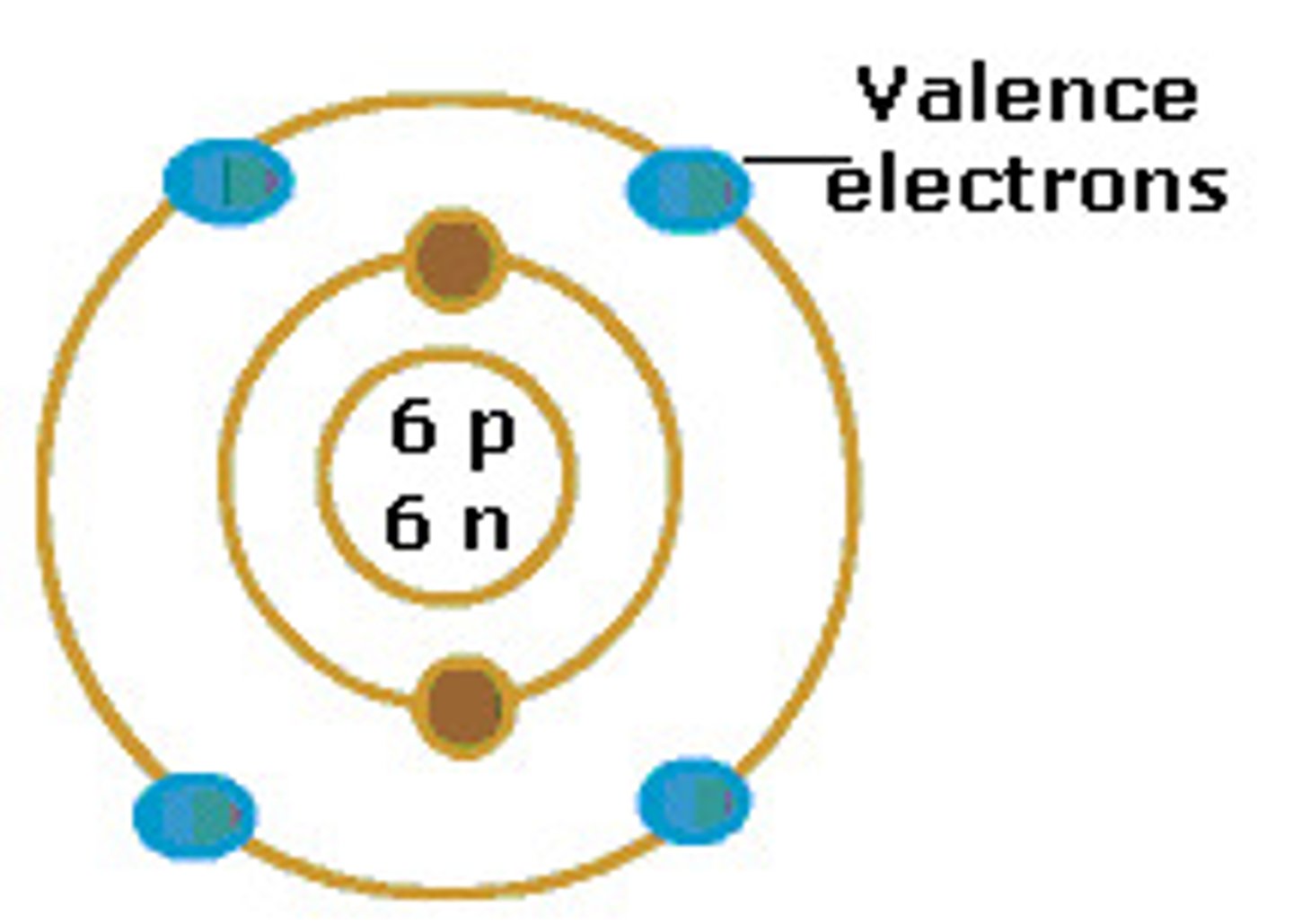
valence electrons
an electron in an outer orbital that can form bonds with atoms.
-they have the highest energy.
-rarely transferred or shared
reactants
in a chemical equation, the substances on the left side of the equation; the starting materials in a chemical reaction.
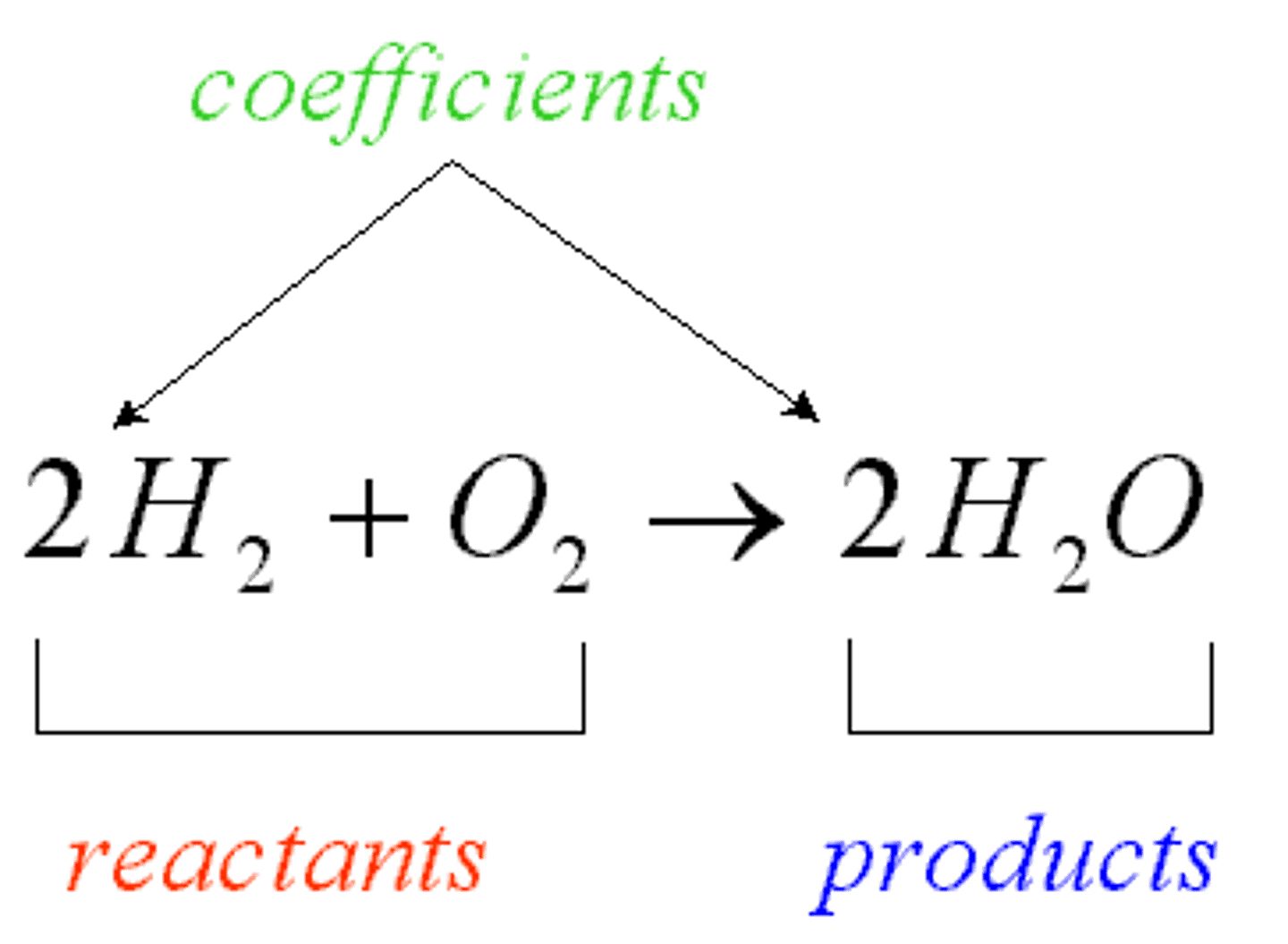
products
in a chemical equation, the substances on the right side of the equation; the substances that are formed in a chemical reaction
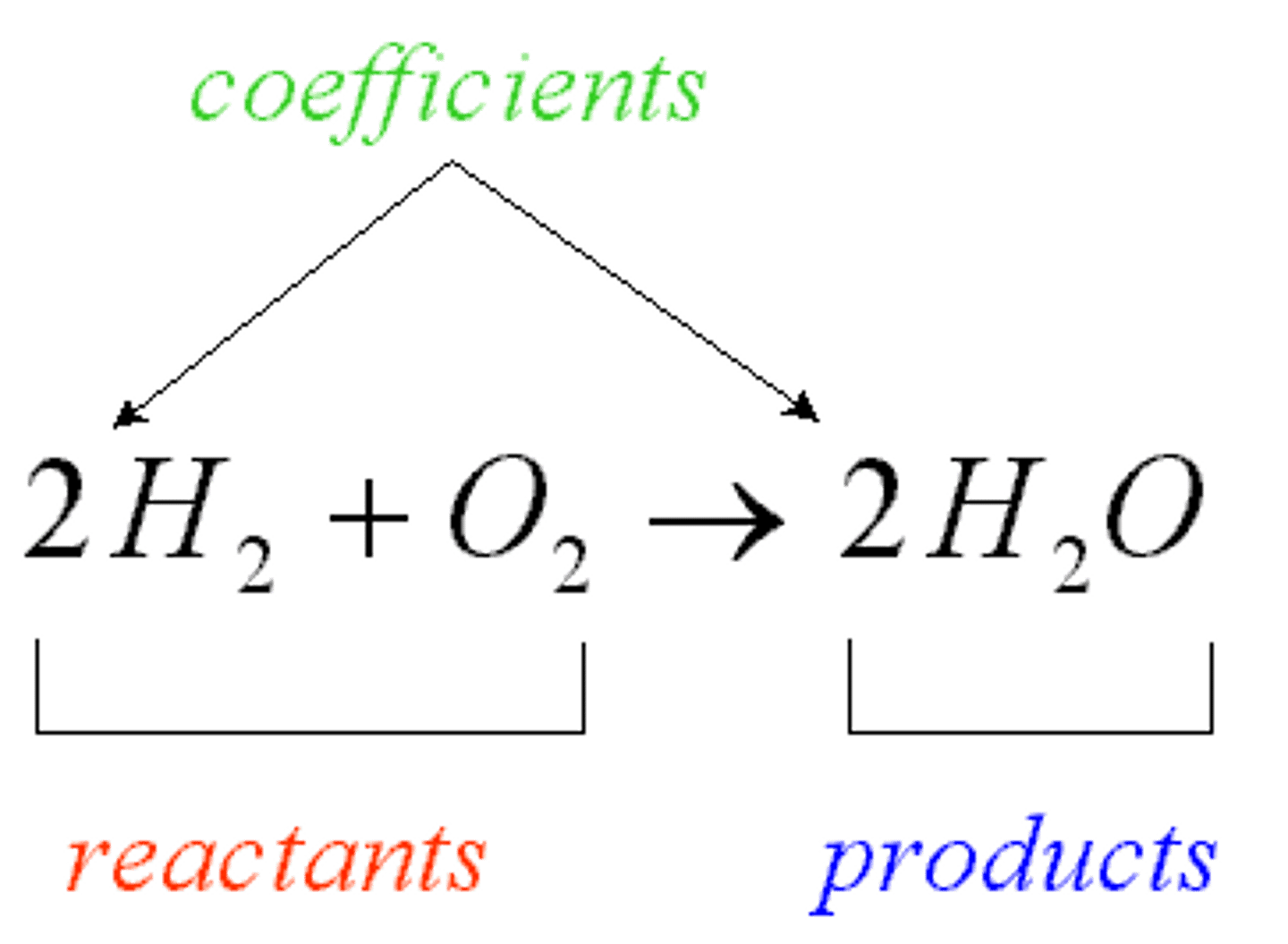
transfers or sharing of an electron with another atom allows
the atoms to achieve the noble gas electron configuration by having eight electrons in the outermost shell and becoming stable.
the number of valence electrons an element has can
be determined based on its group in the periodic table.
it turns out that elements in the same group in the periodic table have the same
chemical behavior because they have the same number of valence electrons.
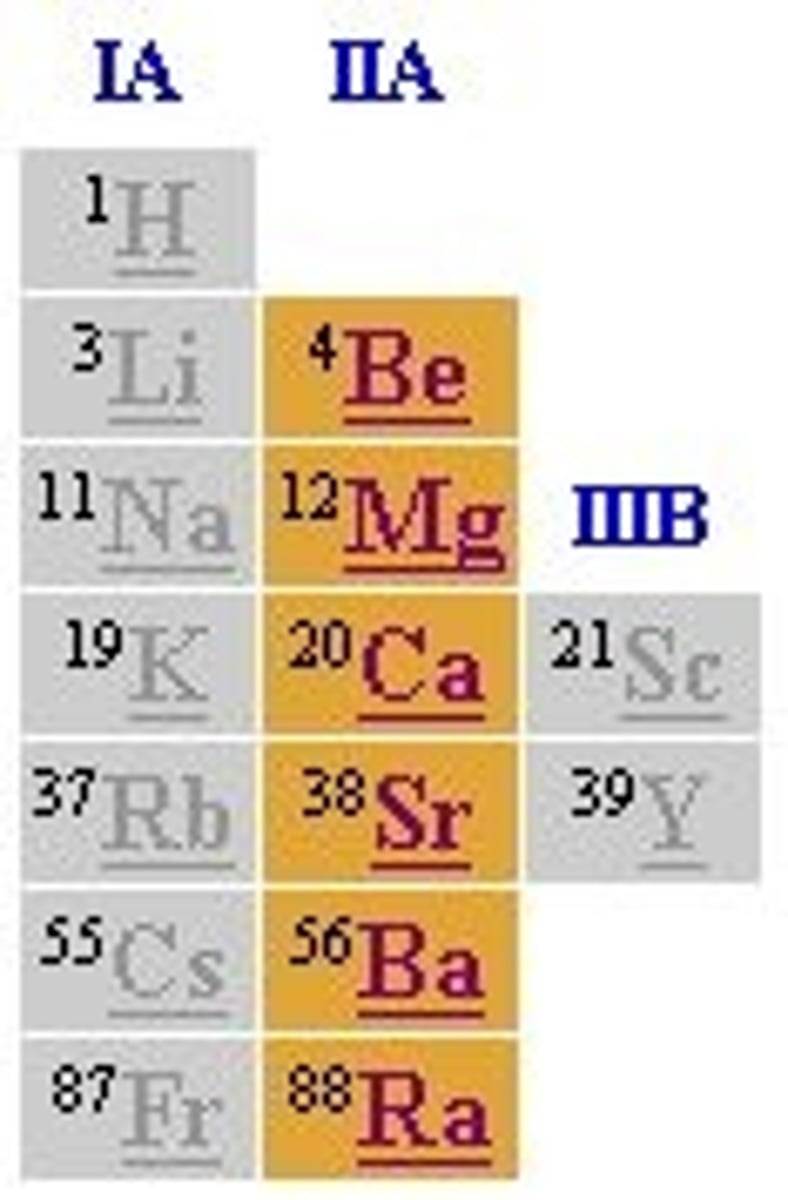
Group IA alkali metals such as sodium and potassium have
one valence electrons
-loses one valence electron, leaving an imbalance between the number of positive protons in the nucleus and electrons as a result of positive cations with a +1 charge with an outermost shell similar to a noble gas.
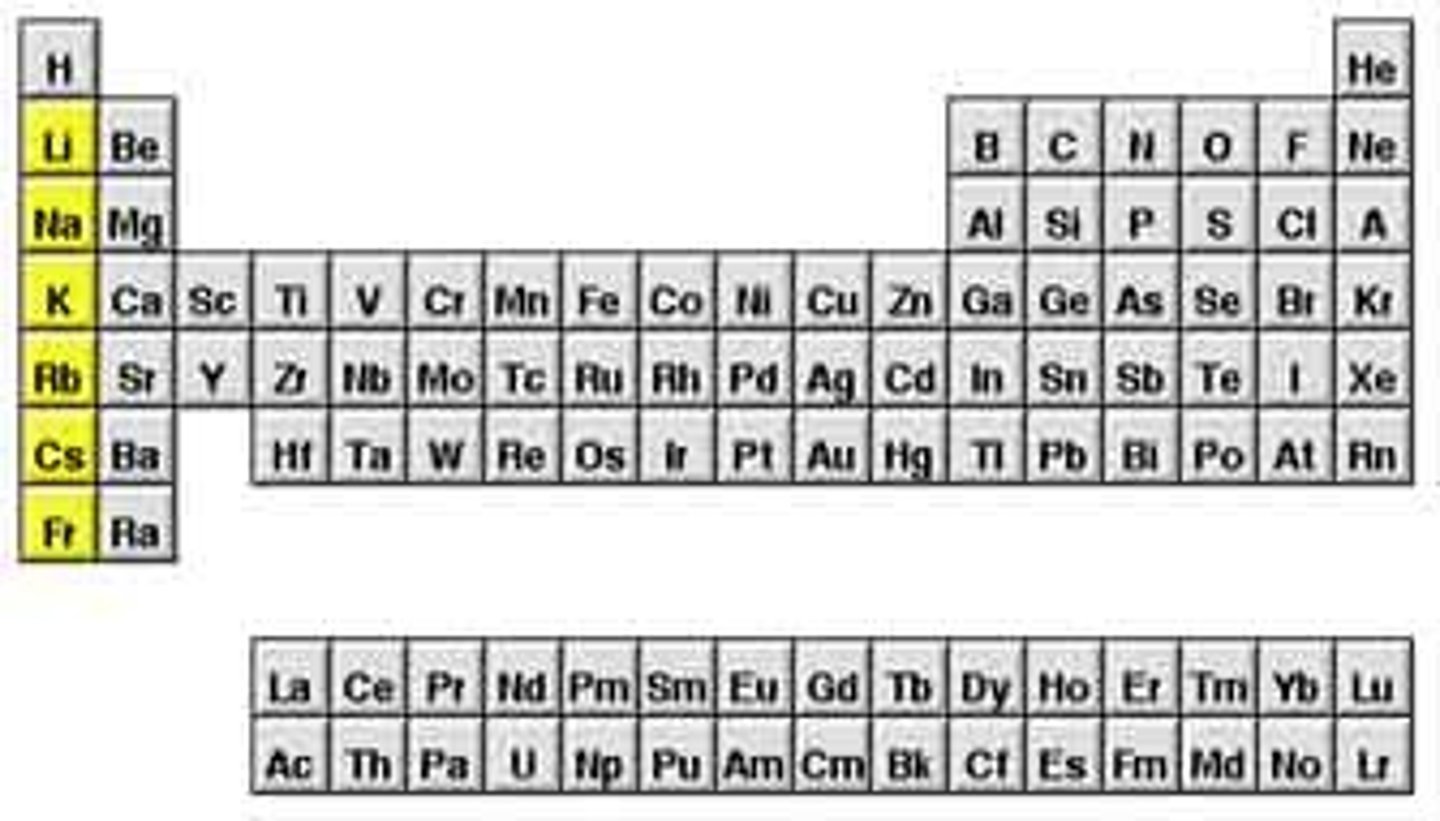
Group IIA: Alkaline Earth Metals such as magnesium and calcium have
two valence electrons
for example, sodium (Na) loses the only electron in its valence shell,
so now the Na+ ion has the same number of electrons as neon (Ne) and therefore is more stable.
for example, magnesium (Mg) can lose valence electrons, forming
Mg2+, which also has the same number and configuration of electrons as Ne.
the elements in the upper right side of the periodic table, such as those in group VIA and VIIA, tend to
gain electrons in chemical reactions.
-these elements have ionization energies and significant ability to draw valence electrons from other atoms towards themselves.
elements in Group VIIA, such as fluorine (F), chlorine (Cl), and bromine (Br) gain
one electrons and thus end up with more electrons than the number of protons in the nucleus
-they make negative anions with a - 1 charge (F-, Cl-, Br-)
because Group VIIA end up with the same number of electrons as the neighboring noble gas,
they achieve stability.
Group VIA elements oxygen (O) and sulfur (S) gain 2 electrons,
forming O2- and S2- ions and noble gas valence electron configurations for stability
Elements on the left side of the periodic table with low ionization energies and low electronegativity are
metals, which will tend to lose electrons and become cations.
the electrons lost by metals are
gained by elements on the right side of the periodic table, which have high electronegativities that is high; a high affinity for them and are nonmetals
when two or more nonmetals react,
they become stable by sharing valence electrons.
nonmetals gain electrons to
become negatively charged anions.
-found on the right side of the periodic table
chemical bonds occur when
when two or more atoms have interactions between their valence electrons.
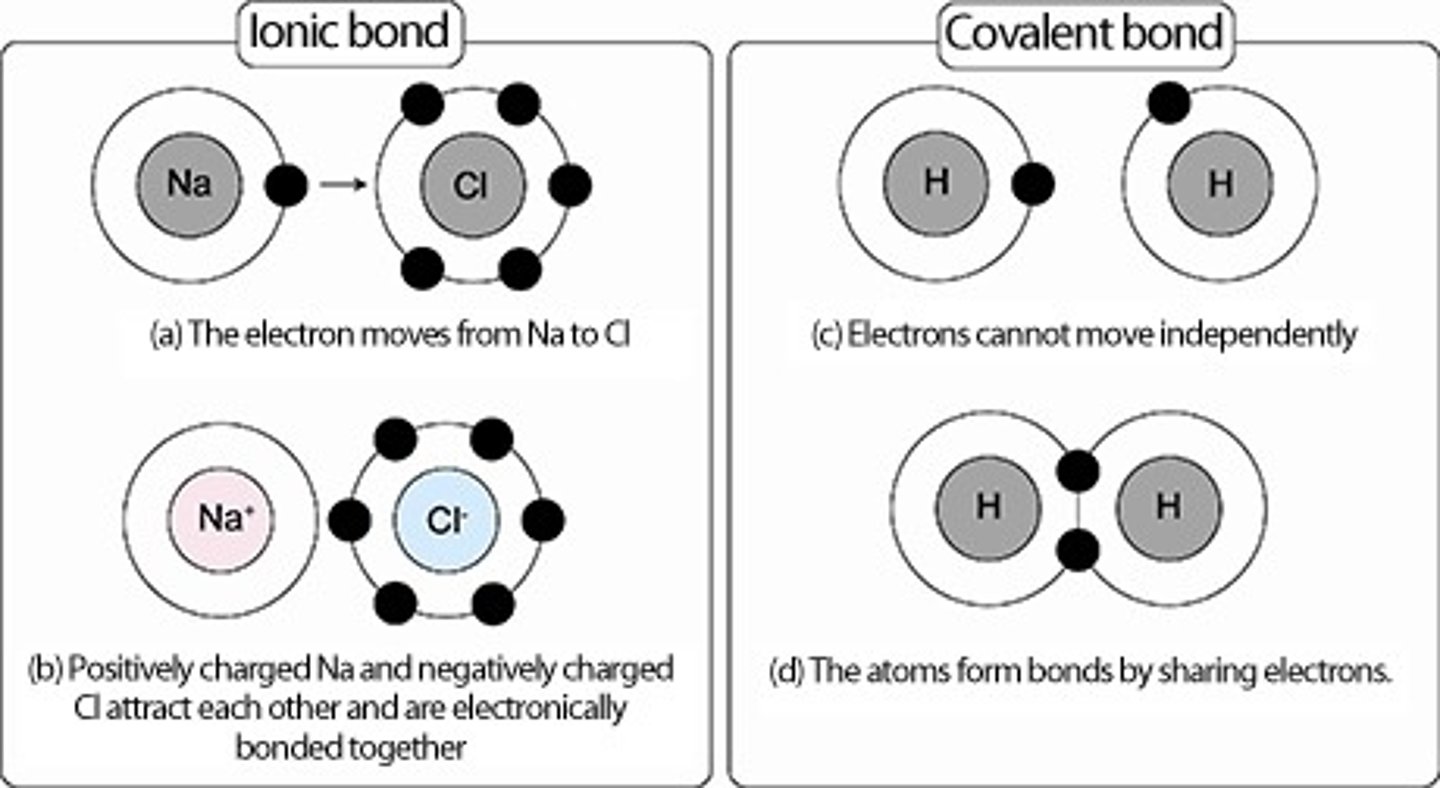
ionic bond
can only form when the elements involved have a large difference in electronegativity such as exists between metals and nonmetals
metals tend to
lose electrons to form positive ions called cations.
-found on the left side of the periodic table
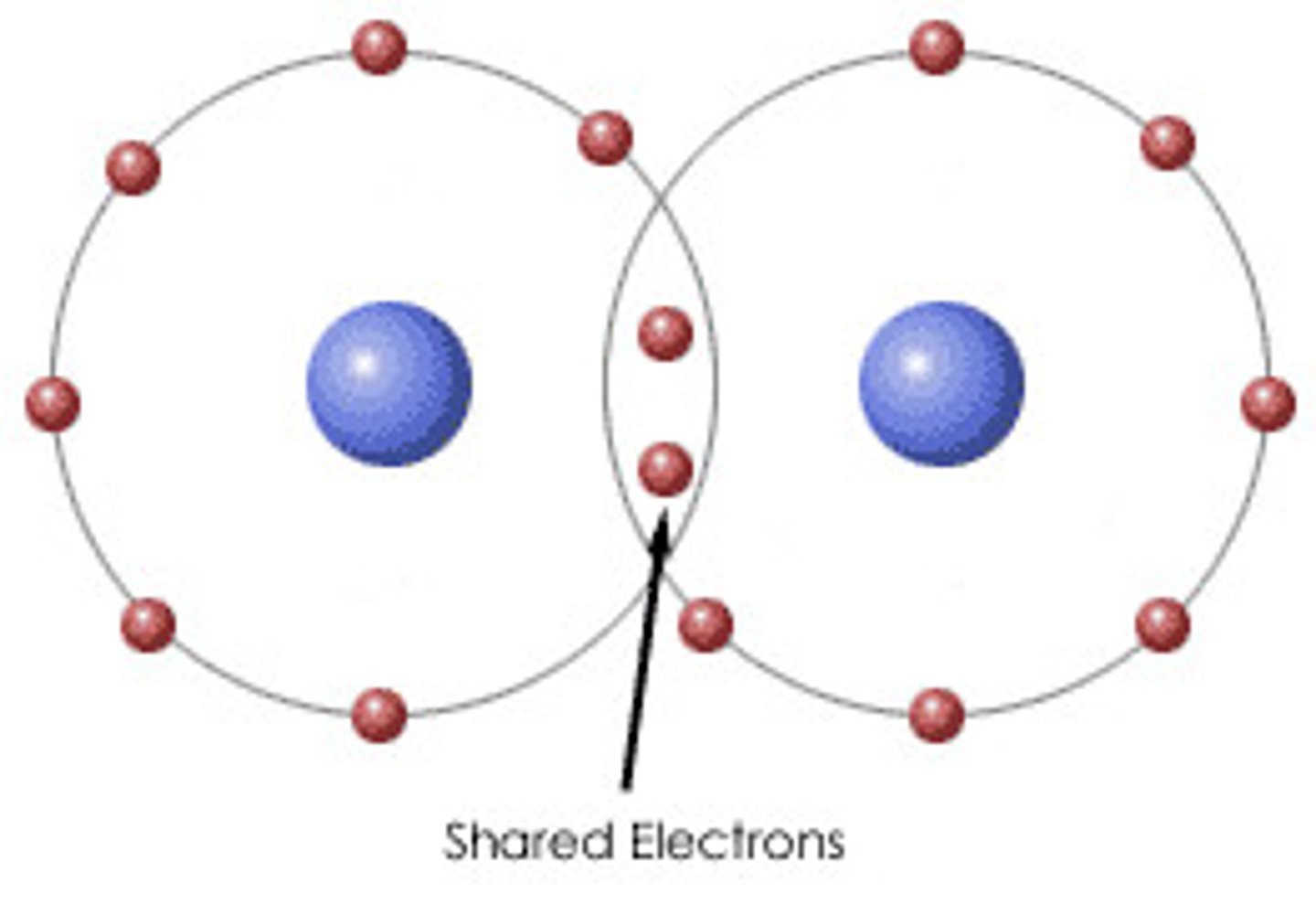
covalent bond
A chemical bond is formed when two atoms share electrons
-no sufficient difference in electronegativity to gain or lose electrons.
polar covalent bond
A covalent bond between atoms that differ in electronegativity. The shared electrons are pulled closer to the more electronegative atom, making it slightly negative and the other atom slightly positive.
-(unequal sharing of electrons)
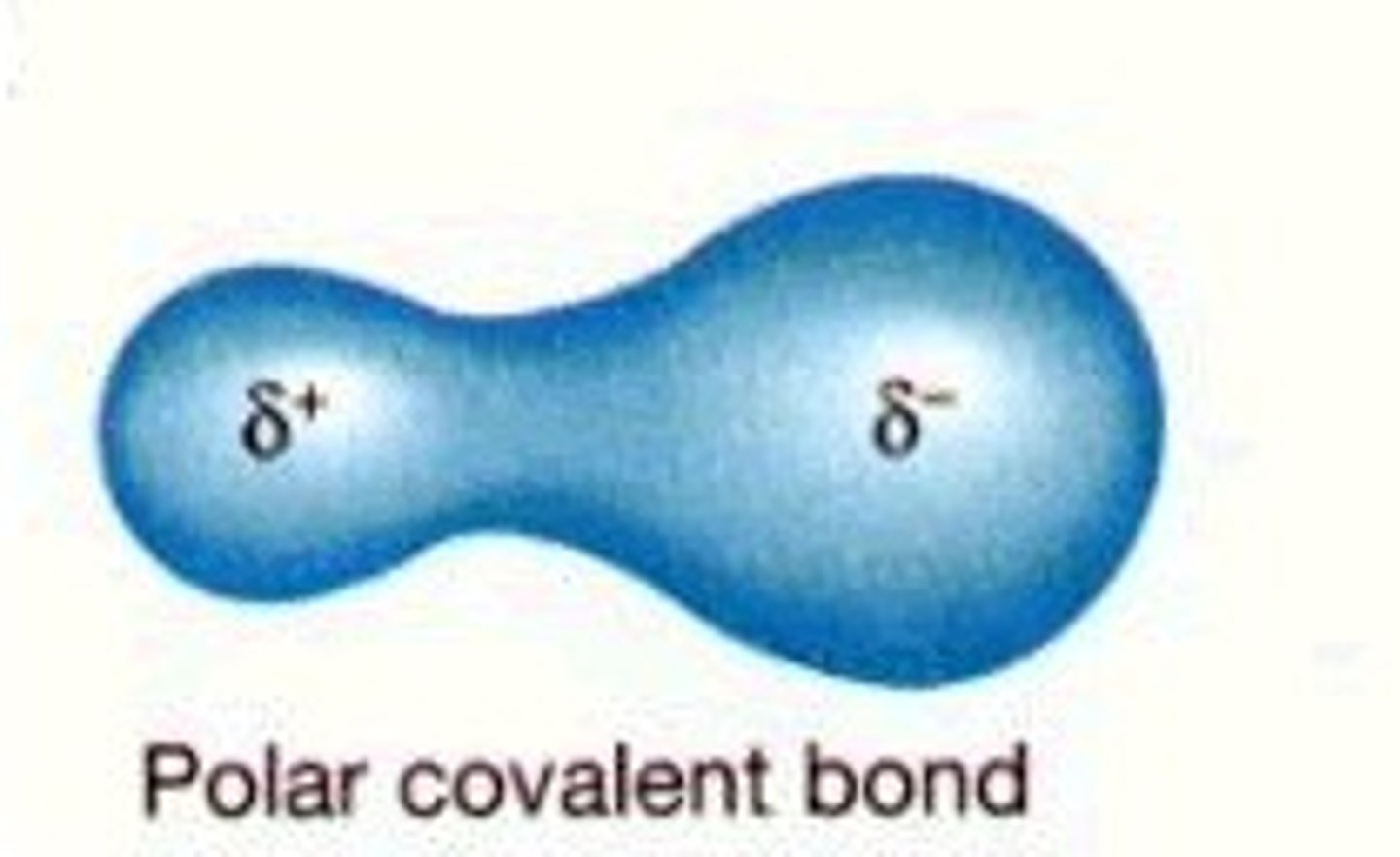
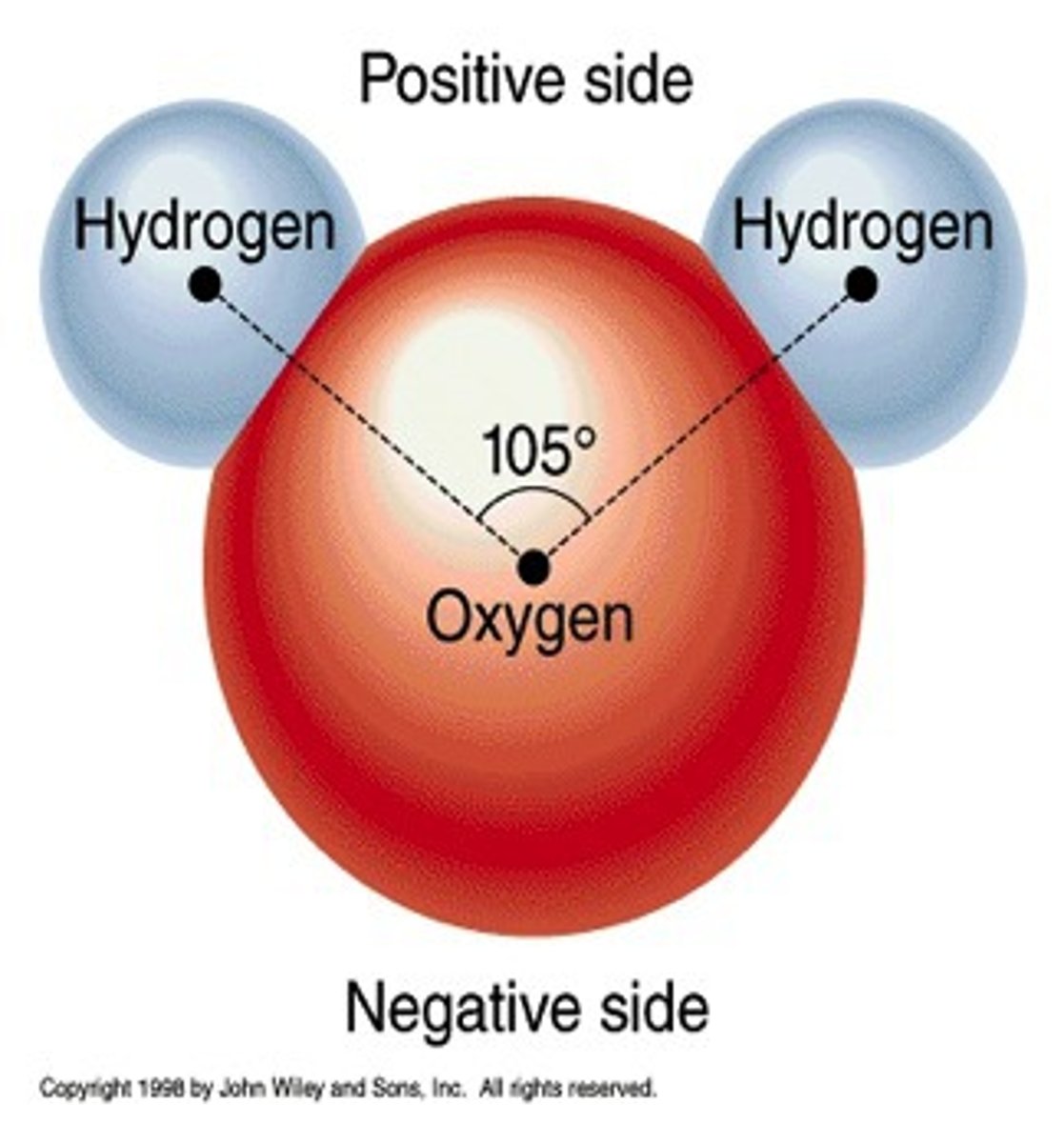
water is a
polar molecule

hydrogen side of the molecule is
partially positively charged
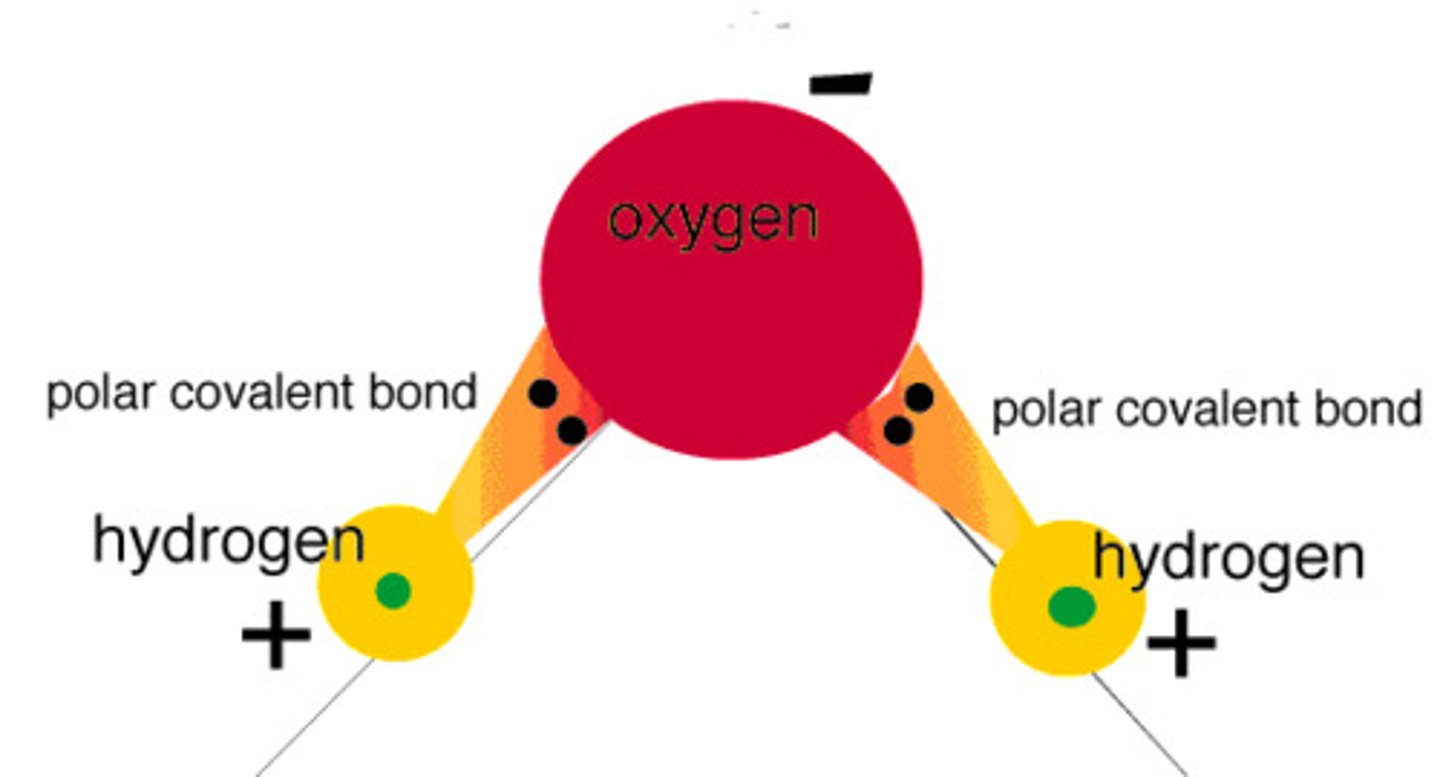
oxygen side is
partially negatively charged.
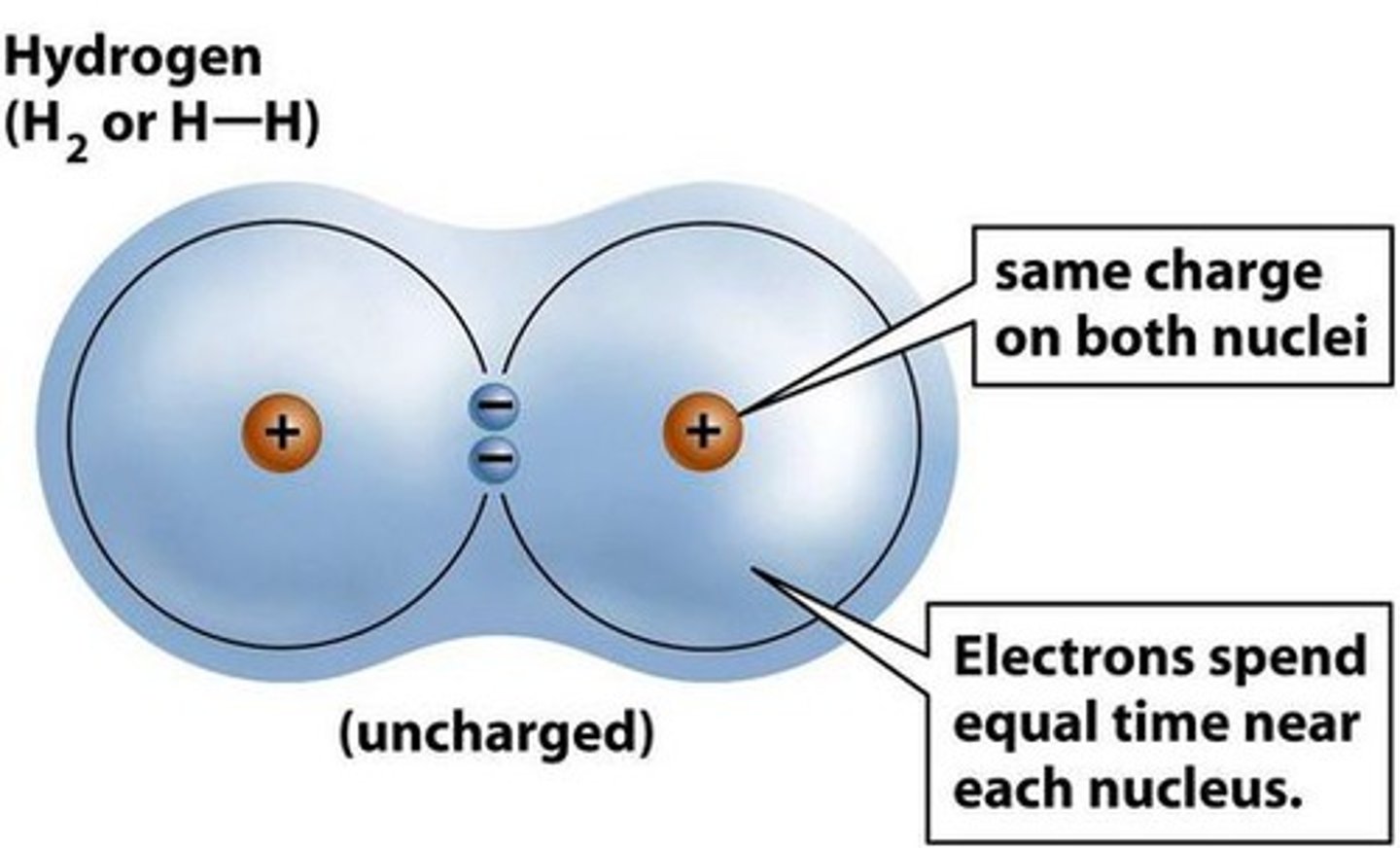
nonpolar covalent bond
a covalent bond in which the electrons are shared equally by the two atoms
-equal sharing of electrons
chemical equations
mathematical representation of a chemical reaction.
-basic layout going from left to right: reaction sign showing the direction of the reaction (->), and products.
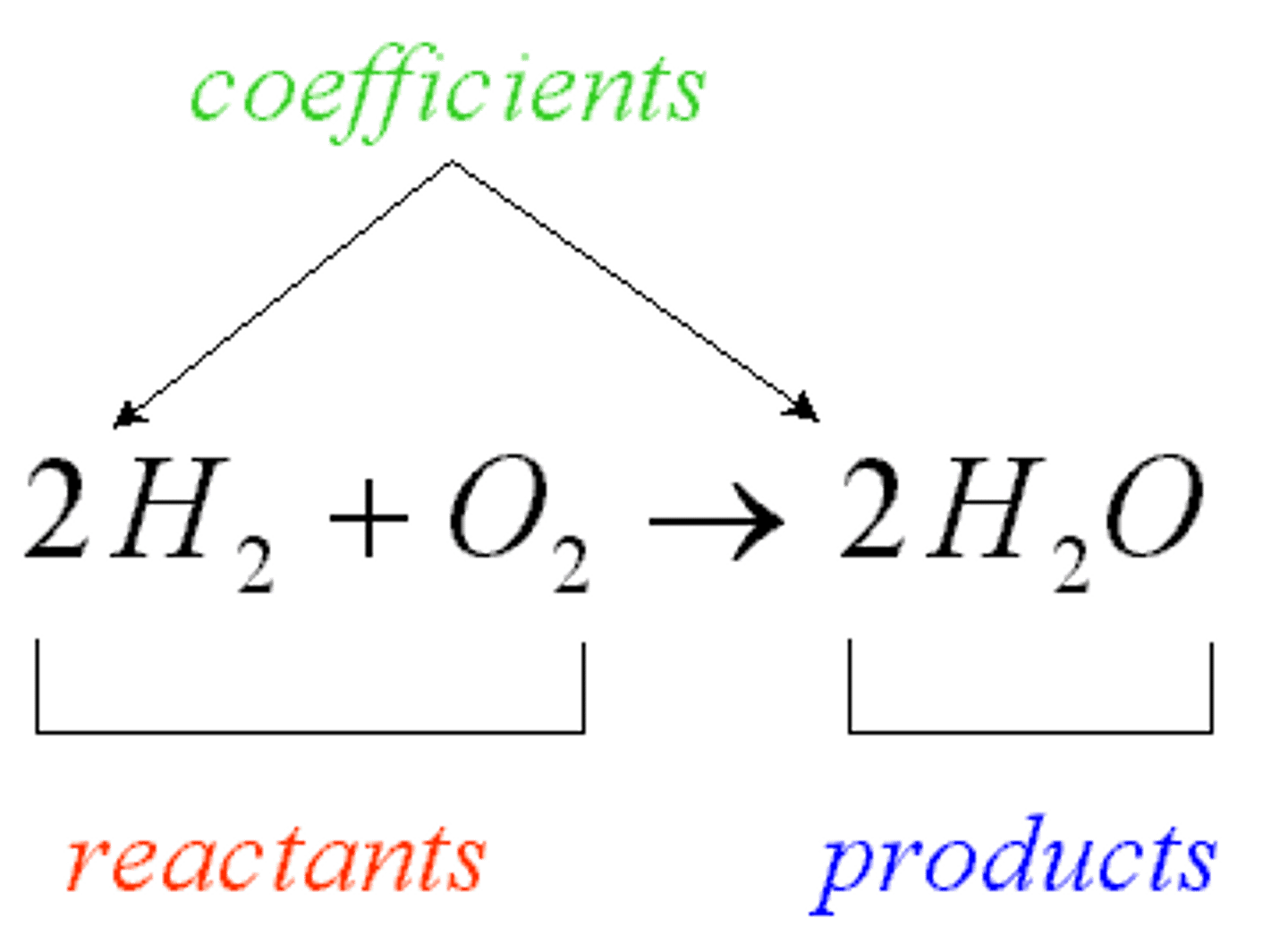
YOUTUBE CHEMICAL REACTION
PRACTICE QUESTIONS FOR CHEMICAL REACTION!!!
YOUTUBE OF BALANCING CHEMICAL REACTION
PRACTICE QUESTION FOR BALANCING CHEMICAL REACTION!!!
mole
a unit of a substance that is equal to exactly 6.02214076x10>23 particles of that substance
-atomic mass (measured in grams) of a substance.

YOUTUBE MOLE CHEMICAL REACTIONS EQUATION
PRACTICE MOLE CHEMICAL REACTION EQUATIONS!!!