2. BIOLOGICAL MOLECULES
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
64 Terms
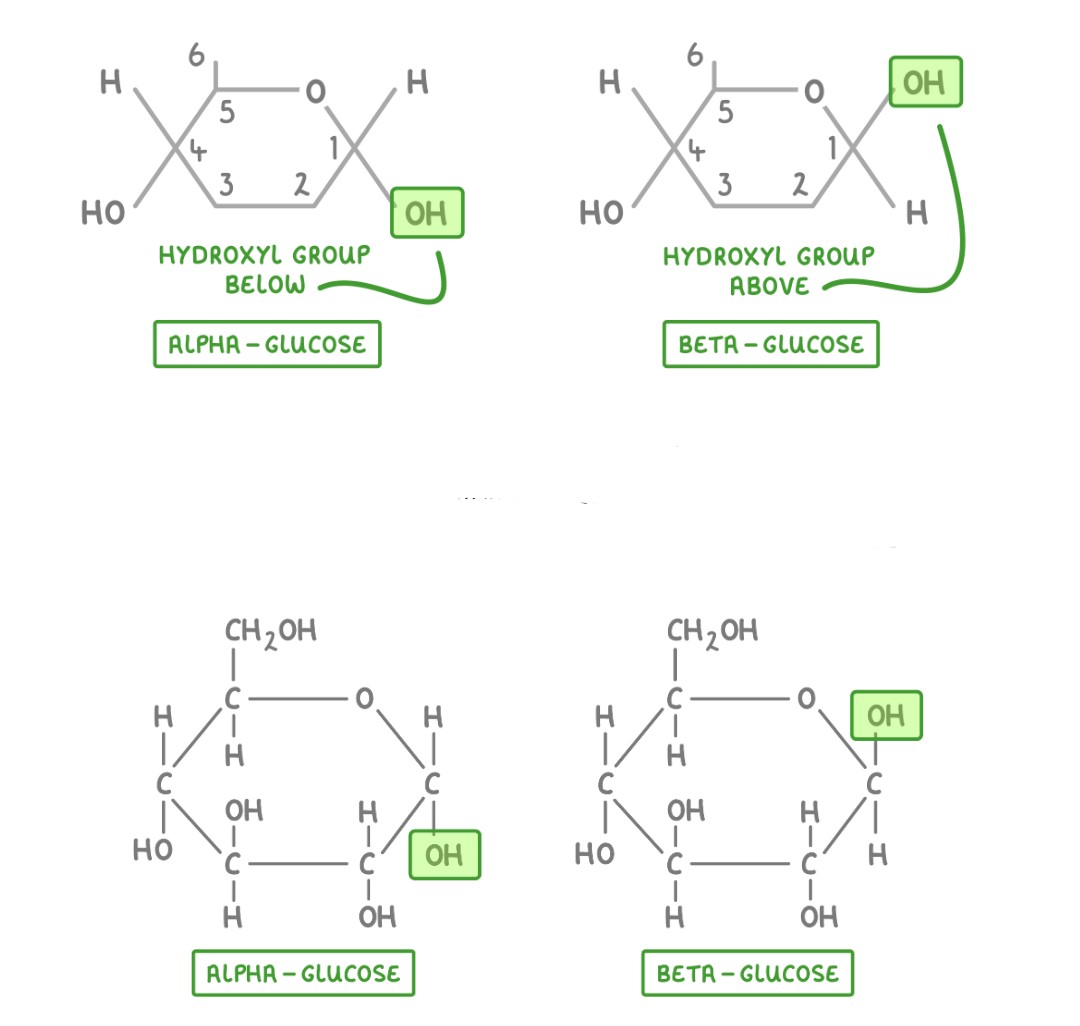
Alpha-glucose and Beta glucose structures and result of structural variety
- α-glucose → Forms starch & glycogen → Compact & efficient for energy storage → Easily digestible in animals.
- β-glucose → Forms cellulose → Strong, rigid fibers for plant cell walls → Not digestible by humans but broken down by specialized microbes in herbivores.
Why are carbohydrates key organic compounds
Each carbon atom can form four covalent bonds – this makes the compounds very stable (as covalent bonds are so strong they require a large input of energy to break them)
Carbon atoms can form covalent bonds with oxygen, nitrogen and sulfur
Carbon atoms can bond to form straight chains, branched chains or rings
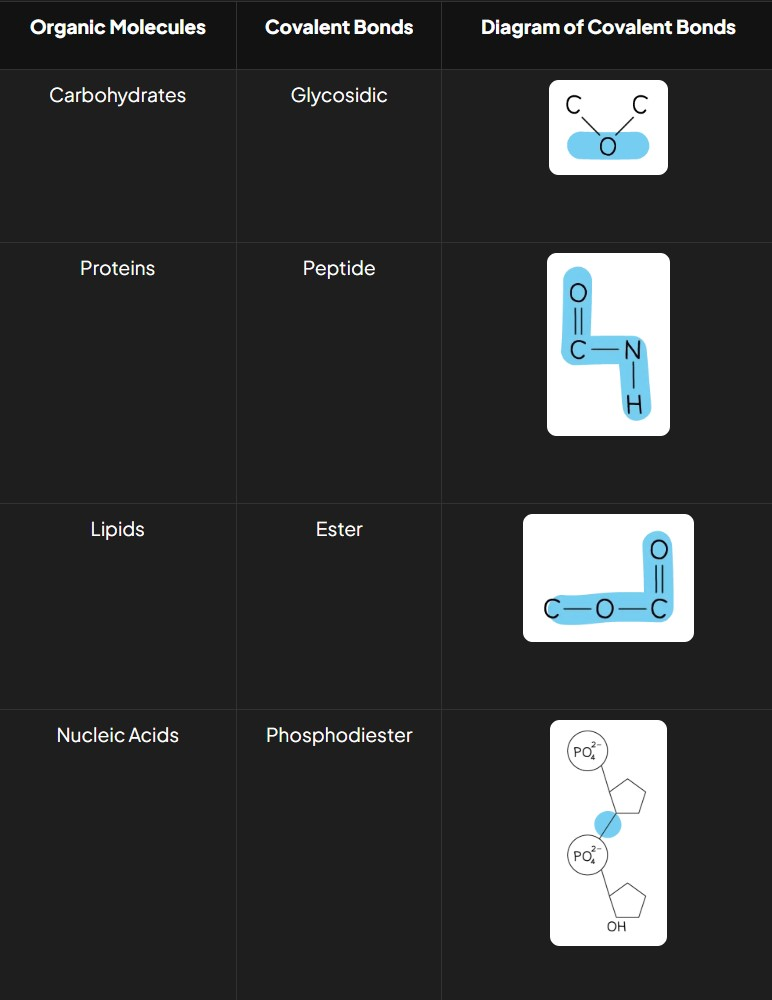
What are the types of covalent bonds in organic molecules and what do they look like?
Carbohydrates: Glycosidic bonds
Proteins: Peptide bonds
Lipids: Ester bonds
Nucleic Acids: Phosphodiester bonds
Why are monosaccharides bonded to form disaccharides and polysaccharides?
Bonding monosaccharides reduces their influence on a cell’s osmolarity, making them more suitable for transport and storage.
How does the type of glycosidic bond vary in carbohydrates?
Different monosaccharides form specific glycosidic bonds:
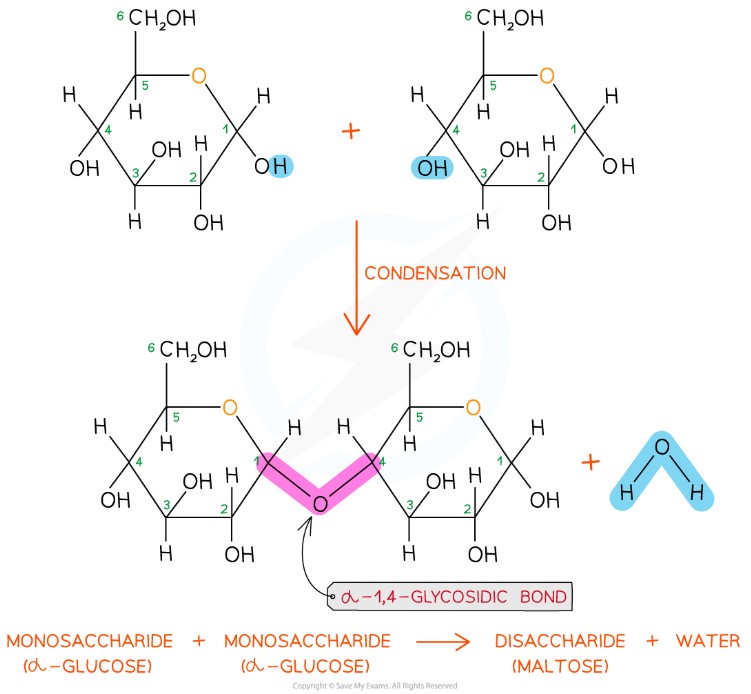
Maltose: α-1,4 bond (disaccharide)
Sucrose: α-1,2 bond (disaccharide)
Cellulose: β-1,4 bond (polysaccharide)
Amylose: α-1,4 bond (polysaccharide)
Amylopectin: α-1,4 and α-1,6 bonds (polysaccharide)
Bonding in maltose
bonding in cellulose
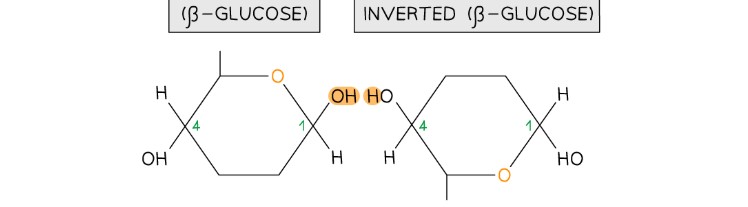
How is sucrose formed via glycosidic bonding?
Sucrose is formed when α-glucose and β-fructose join via a condensation reaction, resulting in an α-1,2 glycosidic bond.
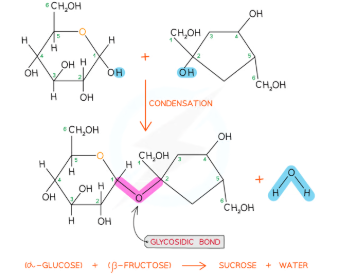
1,6 Glycosidic bonds
ONLY FORM ON BRANCHES
Why are starch and glycogen ideal as storage polysaccharides?
Both are compact (allowing many molecules to fit in small spaces) and insoluble (preventing osmotic imbalance in cells, which soluble molecules like glucose could cause).
What is starch and where is it stored in plants?
Starch is the primary storage polysaccharide in plants, stored as granules in plastids, such as chloroplasts. It consists of two types of polysaccharides: amylose and amylopectin
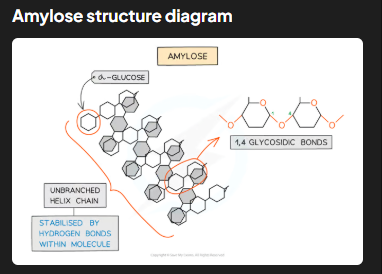
Describe the structure and function of amylose in starch
Amylose makes up 10-30% of starch. It is an unbranched, helix-shaped polymer of α-glucose linked by 1,4 glycosidic bonds. The helical structure makes amylose compact and resistant to digestion, enabling efficient energy storage in plants
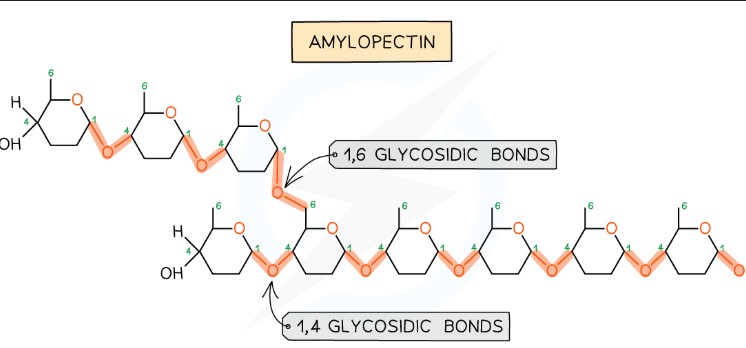
Describe the structure and function of amylopectin in starch.
Amylopectin accounts for 70-90% of starch. It has 1,4 glycosidic bonds but also 1,6 glycosidic bonds, forming a branched structure. Branching provides many terminal glucose molecules, which are easily hydrolyzed during cellular respiration for quick energy release.
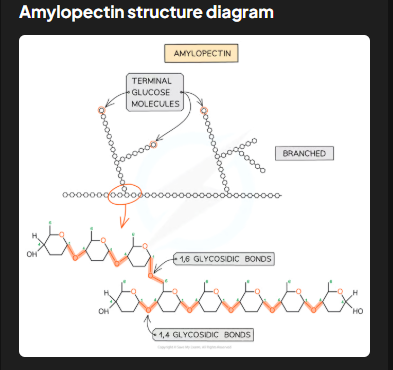
How does amylopectin's structure relate to its digestibility?
The branched structure allows for faster hydrolysis of terminal glucose molecules, ensuring energy can be quickly accessed or added for storage, depending on cellular needs.
What is glycogen and where is it predominantly found?
Glycogen is the storage polysaccharide of animals and fungi. It is stored as visible granules in liver and muscle cells, where the demand for glucose due to high cellular respiration rates is significant.
How does glycogen's structure compare to amylopectin's?
Glycogen is even more branched than amylopectin. This extra branching makes glycogen more compact, enabling animals to store larger amounts of glucose in smaller spaces.
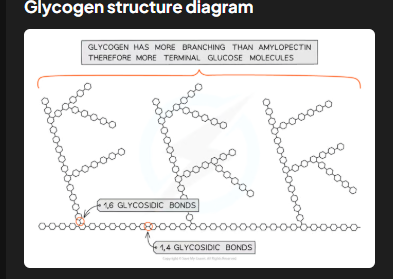
Why does glycogen's highly branched structure suit animal storage needs?
The numerous free ends facilitate rapid addition or removal of glucose molecules through condensation or hydrolysis reactions, allowing animals to quickly store or release glucose based on their energy demands.
How does the insolubility of starch and glycogen benefit living organisms?
Starch and glycogen are insoluble polysaccharides, which makes them ideal for storage in living organisms due to the following reasons:
Avoidance of Osmotic Effects: Being insoluble means they do not dissolve in the cytoplasm. If they were soluble like glucose, they would lower the water potential of the cell cytoplasm. This could cause water to enter the cell by osmosis, leading to cell swelling or bursting (lysis). By remaining insoluble, starch and glycogen prevent this osmotic imbalance and maintain cellular integrity.
Efficient Energy Storage: Insolubility ensures that these polysaccharides do not diffuse out of cells, keeping the stored energy readily available within the cell for use when needed.
Maintenance of Metabolic Balance: Since they are non-reactive and insoluble, starch and glycogen do not interfere with other metabolic processes in the cell. This allows them to function solely as storage molecules without disrupting other cellular activities.
Compact Storage: The insoluble nature of starch and glycogen contributes to their compact structure, enabling large quantities to be stored in a small space within cells (e.g., as granules in plastids for starch or in the cytoplasm for glycogen).
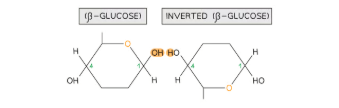
What is cellulose? What is the structure of cellulose, and how does it form?
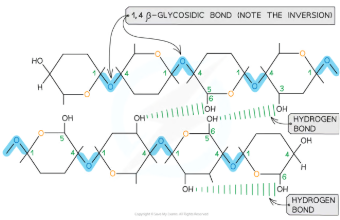
Cellulose is a polysaccharide composed of long chains of β-glucose monomers joined by 1,4 glycosidic bonds. To form these bonds, adjacent β-glucose molecules must rotate 180° relative to one another. This unique arrangement enables the parallel alignment of chains and facilitates the formation of many hydrogen bonds between chains. These hydrogen bonds give cellulose its exceptional strength and stability, making it ideal for structural roles.
How do the parallel chains in cellulose contribute to its mechanical properties?
Parallel chains of cellulose are grouped into bundles known as microfibrils. These microfibrils are tightly packed and extensively connected by hydrogen bonds, which significantly enhance the tensile strength of cellulose. This high tensile strength ensures that cellulose can resist stretching forces, making it capable of withstanding the turgor pressure exerted by water-filled plant cells.
What roles do cellulose microfibrils play in plant cell walls?
The microfibrils form a rigid framework within the cell wall, providing structural support to the plant. They are embedded in a matrix of other substances, such as lignin, which further increases the wall's strength. This composite structure ensures that plant cells can maintain their shape, support the overall plant structure, and resist external mechanical stresses like wind or pressure.
Why is cellulose insoluble, and how does this benefit its role in plants?
Cellulose is insoluble in water due to its extensive hydrogen bonding and crystalline structure. This property prevents it from dissolving or disrupting cell functions, allowing it to maintain its structural integrity within the cell walls. Insolubility also ensures that cellulose does not interfere with the osmotic balance within plant cells.
How does cellulose's structure facilitate its permeability and additional functions?
The arrangement of cellulose fibres leaves spaces within the cell wall, making it freely permeable to water and solutes. This allows efficient exchange of substances, supporting cellular processes such as nutrient transport. Additionally, because few organisms produce the enzyme cellulase needed to digest cellulose, it serves as an indigestible source of dietary fibre for many organisms.
What are triglycerides, and what are their key characteristics?
Triglycerides are non-polar, hydrophobic lipids composed of glycerol and fatty acids. Their insolubility in water ensures they do not cause osmotic effects in cells, making them ideal for energy storage.
Describe the molecular structure of triglycerides.
Triglycerides consist of one glycerol molecule (an alcohol with hydroxyl groups) bonded to three fatty acid molecules (hydrocarbon chains ending with a carboxyl group) by ester bonds. Fatty acids may be saturated (no double bonds, mainly in animal fats) or unsaturated (one or more double bonds, mainly in vegetable oils). Unsaturated fatty acids may further be classified as cis-fatty acids (enzymes metabolize them) or trans-fatty acids (linked to coronary heart disease).
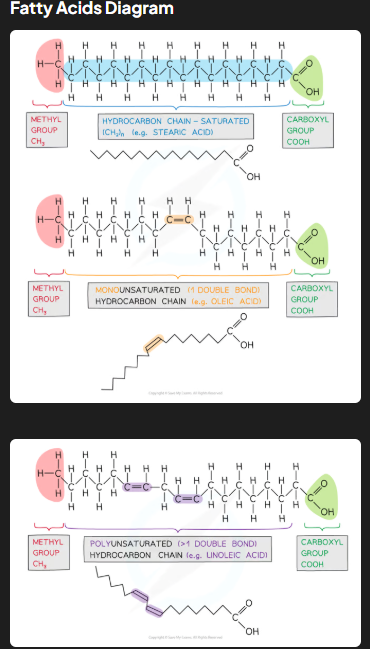
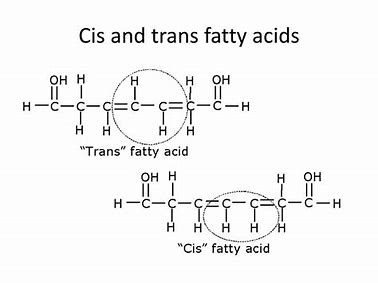
What are mono- and poly-unsaturated as well as cis and trans fatty acids?
Cis-fatty acids: Found in both mono- and poly-unsaturated fatty acids. Hydrogen atoms are on the same side of the double bond, creating a kink in the chain. These fats are typically found in natural oils (e.g., olive oil) and are metabolized efficiently.
Trans-fatty acids: May form during hydrogenation of unsaturated fats. Hydrogen atoms are on opposite sides of the double bond, straightening the chain, allowing tighter packing like saturated fats. Trans-fats are associated with coronary heart disease as they are not metabolized efficiently and can increase harmful cholesterol levels
How does the molecular structure of triglycerides relate to their function in energy storage?
Triglycerides have long hydrocarbon chains with many carbon-hydrogen bonds and little oxygen, enabling them to store more energy per gram (37kJ compared to 17kJ for carbohydrates). When oxidized during cellular respiration, these bonds release energy for ATP production and metabolic water, vital for desert animals and embryos.
What other functions do triglycerides serve in living organisms?
Insulation: Compose the myelin sheath for nerve signal transmission and form an adipose tissue layer below the skin to retain heat.
Buoyancy: Their low density helps aquatic animals like whales float.
Protection: Adipose tissue cushions organs, reducing the risk of damage during movement or impact.
What is the molecular structure of phospholipids?
Phospholipids consist of glycerol, two fatty acid tails, and a phosphate group.
The phosphate head is hydrophilic (polar and water-soluble), while the fatty acid tails are hydrophobic (non-polar and water-insoluble).
This amphipathic nature (having both hydrophilic and hydrophobic parts) allows phospholipids to form bilayers in cell membranes.
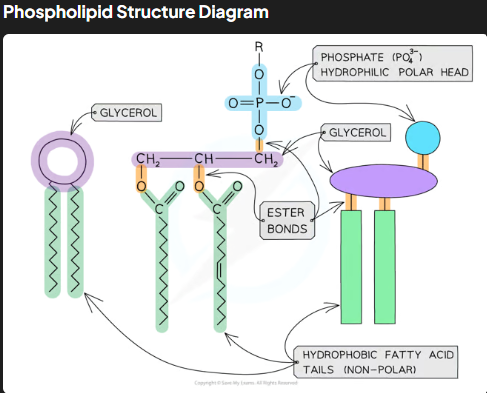
How do phospholipids organize in water, and what is the significance of this arrangement?
In water, phospholipids form monolayers or bilayers due to their amphipathic nature.
In bilayers, hydrophobic tails face inward, creating a hydrophobic core, while hydrophilic heads face outward, interacting with water.
This bilayer structure is crucial for forming the cell membrane, acting as a barrier to water-soluble substances while allowing compartmentalization of cellular functions.
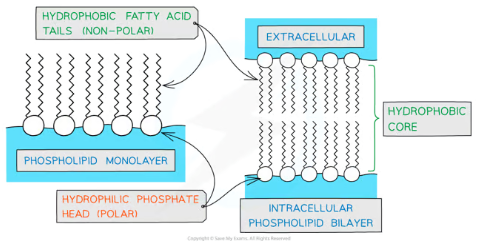
What is the role of phospholipids in cell membranes, and how does their structure relate to this function?
Building Blocks of Membranes:
Phospholipids are the primary component of the cell membrane, forming a bilayer structure.
The hydrophilic phosphate heads face outward, interacting with water inside and outside the cell, while the hydrophobic fatty acid tails face inward, creating a non-polar core.
Barrier Function:
The hydrophobic core acts as a selective barrier, preventing water-soluble (polar) molecules from freely passing through the membrane. This allows the cell to regulate what enters and exits, maintaining homeostasis.
Compartmentalization:
The bilayer creates enclosed environments within cells, enabling organelles to perform specific roles efficiently without interference from other cellular processes.
Membrane Fluidity:
The fatty acid composition affects membrane fluidity:
Unsaturated fatty acid tails: Create kinks, preventing close packing and increasing fluidity, ensuring flexibility and the ability of cells to adapt to temperature changes.
Saturated fatty acid tails: Allow closer packing, reducing fluidity for more rigid structures.
Protein Orientation and Mobility:
Weak hydrophobic interactions between the phospholipids and membrane proteins hold these proteins in place while allowing lateral movement. This mobility ensures proteins can function in processes like cell signaling and transport.
Dynamic Nature:
The amphipathic property of phospholipids ensures the bilayer is both stable and dynamic, capable of self-healing and forming vesicles for transport within the cell.
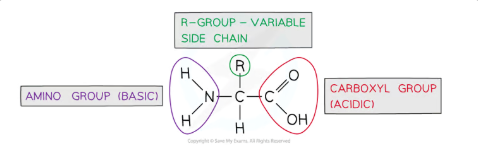
What is the general structure of an amino acid?
Amino acids are monomers of proteins with a central carbon atom bonded to:
Amine group (-NH₂)
Carboxylic acid group (-COOH)
Hydrogen atom (H)
R group, which varies and determines the properties of the amino acid (e.g., polar, non-polar, acidic, basic).
There are 20 amino acids common to all living organisms, differing only in their R groups.
How is a peptide bond formed between amino acids?
A peptide bond is formed through a condensation reaction:
A hydroxyl group (-OH) from the carboxylic acid group of one amino acid combines with a hydrogen atom (H) from the amine group of another amino acid.
This reaction releases water (H₂O) and results in the formation of a covalent bond between the carbon (of -COOH) and the nitrogen (of -NH₂).
The resulting molecule is a dipeptide, and repeated bonding of amino acids forms a polypeptide. Proteins may consist of one or more polypeptide chains.
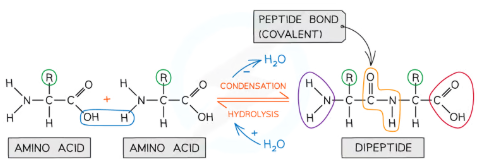
How is a peptide bond broken?
A peptide bond is broken through a hydrolysis reaction:
Water (H₂O) is added to the peptide bond, causing the bond to break.
The amino acids are separated, with the hydroxyl group (-OH) attaching to the carboxylic acid group and the hydrogen atom (H) attaching to the amine group.
What are the key features of a peptide bond?
A peptide bond is a covalent bond formed between the carbon (C) of the carboxylic acid group of one amino acid and the nitrogen (N) of the amine group of another.
The R groups of amino acids do not participate in the formation of the peptide bond.
In a protein structure, peptide bonds contribute to the primary structure, creating a linear sequence of amino acids.
What is the primary structure of a protein?
Definition: The sequence of amino acids in a polypeptide chain, linked by covalent peptide bonds.
Determination: The DNA in a cell determines the sequence and quantity of amino acids, which dictates the protein's shape and function.
Specificity: Alterations in the sequence (even one amino acid change) can significantly affect the protein’s function.
Importance: Forms the basis for all higher levels of protein structure.
What is the secondary structure of a protein?
Definition: The structure formed when hydrogen bonds develop between the amino (NH) and carboxyl (CO) groups in the protein backbone.
Types:
α-helix: Hydrogen bonds form every fourth peptide bond, creating a coiled shape.
β-pleated sheet: The polypeptide chain folds, aligning parallel segments held together by hydrogen bonds.
Characteristics: Common in fibrous proteins like collagen and keratin.
Stability: Can be disrupted by high temperatures or pH changes.
What is the tertiary structure of a protein?
Definition: The 3D conformation formed when additional bonds develop between the R groups (side chains) of amino acids in a polypeptide.
Types of Bonds:
Hydrogen bonds: Between polar R groups.
Disulfide bonds: Covalent bonds between sulfur atoms in cysteine amino acids.
Ionic bonds: Between charged R groups.
Hydrophobic interactions: Between non-polar R groups.
Function: Stabilizes the structure of globular proteins, such as enzymes and antibodies.
What is the quaternary structure of a protein?
Definition: The structure formed when two or more polypeptide chains (subunits) work together as a functional macromolecule.
Examples:
Hemoglobin: Contains four polypeptide subunits that collaborate to carry oxygen.
Importance: Adds complexity and functionality to proteins, enabling them to carry out specialized roles.
What are hydrophobic interactions, and how do they contribute to protein structure?
Definition: Hydrophobic interactions occur between non-polar R groups of amino acids within a protein.
Role: These interactions cause hydrophobic R groups to cluster towards the interior of the protein, away from water, helping stabilize the protein's three-dimensional shape.
Location: Found predominantly in the interior of globular proteins.
What are hydrogen bonds, and how do they contribute to protein structure?
Definition: Hydrogen bonds form between strongly polar R groups, such as between -OH or -NH groups.
Role: Though weak individually, hydrogen bonds are abundant and provide significant stabilization to the protein's shape.
Commonality: The most frequent type of bond in proteins, contributing to both secondary (e.g., α-helix, β-pleated sheet) and tertiary structures.
What are ionic bonds, and how do they contribute to protein structure?
Definition: Ionic bonds form between positively charged R groups (e.g., -NH₃⁺) and negatively charged R groups (e.g., -COO⁻).
Role: These bonds are stronger than hydrogen bonds but less common.
Stability: Ionic bonds stabilize tertiary structures but can be broken by changes in pH, which alter the charges of R groups.
What are covalent bonds, including disulfide bonds, and how do they contribute to protein structure?
Definition: Covalent bonds are strong bonds involving the sharing of electrons. Disulfide bonds are a type of covalent bond that occurs specifically between two cysteine amino acids, forming a disulfide bridge (-S-S-).
Role: Disulfide bonds provide extra stability to proteins, especially those secreted from cells, like insulin.
Strength: The strongest interaction in proteins, but less frequent compared to other types of bonds.
Breakage: Can be disrupted by reduction reactions.
How do different interactions collectively maintain protein shape?
Hydrophobic interactions: Cluster non-polar R groups internally to stabilize the structure.
Hydrogen bonds: Form extensively between polar groups, providing flexibility and stability.
Ionic bonds: Link charged R groups to stabilize the structure, sensitive to pH changes.
Disulfide bonds: Reinforce structural integrity, particularly in extracellular proteins.
These interactions together create the tertiary structure, enabling proteins to fold into their functional configurations.
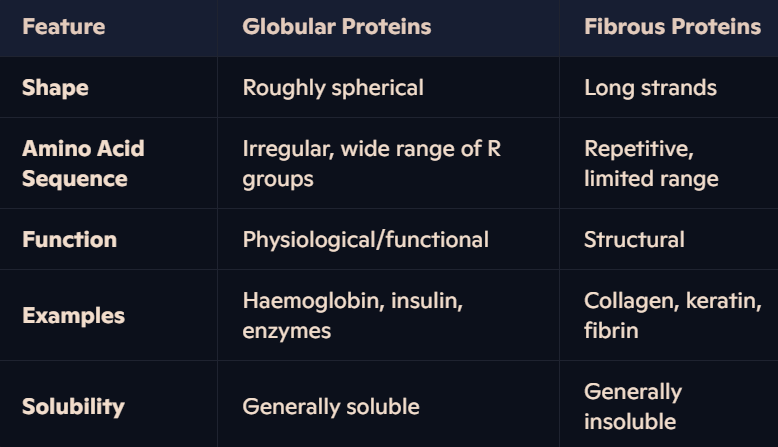
What are globular proteins, and why are they soluble?
Shape: Compact, roughly spherical.
Structure: Tertiary folding orients non-polar hydrophobic R groups inward (away from water) and polar hydrophilic R groups outward.
Solubility: Hydrophilic R groups interact with water, making globular proteins generally soluble.
Function: Solubility enables them to participate in physiological roles, such as metabolic reactions and transport.
What physiological roles do globular proteins perform?
They have specific shapes that allow them to perform critical roles such as:
Enzymes: Catalyze biochemical reactions (e.g., amylase).
Hormones: Regulate physiological processes (e.g., insulin).
Immunoglobulins: Respond to specific antigens in the immune system.
Transport Proteins: Carry substances (e.g., haemoglobin transports oxygen).
Some globular proteins are conjugated proteins, meaning they contain a prosthetic group, e.g., haemoglobin with its haem group.
What are fibrous proteins, and why are they insoluble?
Shape: Long strands of polypeptide chains with cross-linkages.
Amino Acid Sequence: Highly repetitive, forming structured and organized chains.
Structure: High hydrophobic R group content makes them insoluble in water.
Limited Folding: Little or no tertiary structure, contributing to their insolubility.
What structural roles do fibrous proteins perform?
They provide strength and rigidity, making them ideal for structural roles:
Collagen: Found in connective tissues like tendons, skin, and ligaments.
Keratin: Present in hair, nails, horns, and feathers.
Fibrin: Involved in blood clotting.
Their insolubility and organized structure make them resistant to deformation.
How can you compare globular and fibrous proteins?
What is the structure of haemoglobin, and what type of protein is it?
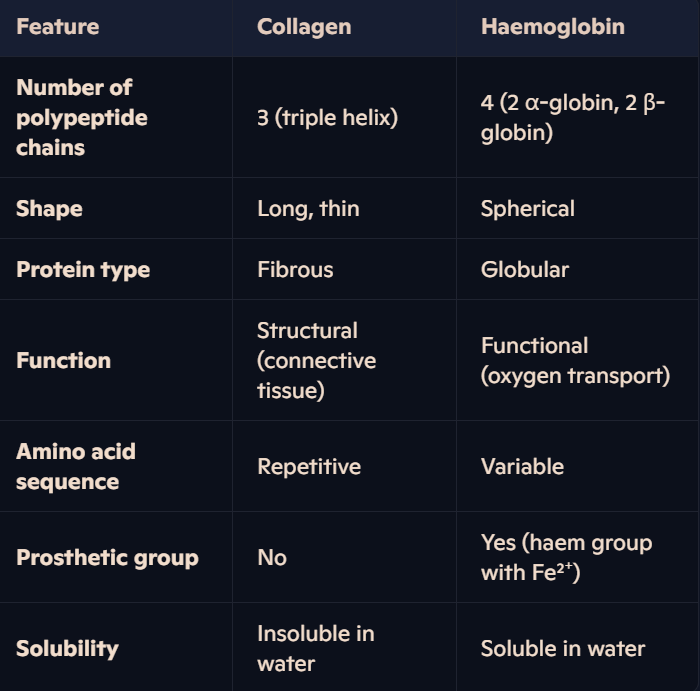
Haemoglobin is a globular protein and a crucial oxygen-carrying pigment found in red blood cells.
Quaternary structure: Comprised of four polypeptide chains (subunits):
Two α-globin subunits and two β-globin subunits.
Each subunit is bound to a prosthetic haem group containing an iron (Fe²⁺) ion.
Subunits are held together by disulphide bonds, and the structure is stabilized by interactions of R groups:
Hydrophobic R groups face inward, maintaining a spherical shape.
Hydrophilic R groups face outward, ensuring solubility in water.
How does the arrangement of haemoglobin’s R groups relate to its solubility and stability?
The inward orientation of hydrophobic R groups preserves its compact, spherical structure.
The outward-facing hydrophilic R groups interact with water, making haemoglobin soluble—essential for efficient transport in the bloodstream.
Changes to the amino acid sequence in globin subunits can disrupt this arrangement, as seen in diseases like sickle cell anaemia.
What is the role of the haem group in haemoglobin?
Each haem group contains an iron II ion (Fe²⁺) at its core, enabling oxygen to bind reversibly.
Haemoglobin can carry up to four oxygen molecules (8 atoms) (one molecule per haem group), forming oxyhaemoglobin, which gives blood its bright red color.
The reversible nature of oxygen binding allows oxygen to be delivered efficiently to tissues for aerobic respiration.
How does the structure of haemoglobin enable efficient oxygen transport?
The solubility of haemoglobin allows oxygen, which is poorly soluble in water, to be transported efficiently.
Binding of the first oxygen molecule induces a conformational change in haemoglobin's quaternary structure, increasing the protein's affinity for subsequent oxygen molecules (cooperative binding).
This mechanism ensures efficient oxygen uptake in the lungs and release in tissues.
Why is iron (Fe²⁺) in the haem group essential for haemoglobin’s function?
The Fe²⁺ ion in the haem group binds oxygen reversibly, a property critical for oxygen transport.
Without Fe²⁺, the haem group could not bind oxygen, as none of the amino acids in haemoglobin’s polypeptide chains are suitable for this function.
The iron ion’s role is crucial for forming oxyhaemoglobin, ensuring oxygen delivery to tissues and removal of carbon dioxide.
What is collagen, and where is it found?
Collagen is the most common fibrous structural protein in vertebrates.
It forms the major component of connective tissue found in:
Tendons, cartilage, ligaments, bones, teeth, skin, blood vessel walls, and the cornea of the eye.
Collagen is insoluble in water, making it ideal for structural roles.
What is the molecular structure of collagen?
Collagen consists of three polypeptide chains, held together by hydrogen bonds to form a triple helix, called tropocollagen.
Each polypeptide chain contains around 1,000 amino acids with a high proportion of glycine, proline, and hydroxyproline.
Glycine (the smallest amino acid) appears in nearly every third position in the primary sequence.
Its small R group (a single hydrogen atom) allows the tight packing of the three chains within the triple helix.
Hydrogen bonds and covalent bonds stabilize the helix. Covalent bonds between R groups of adjacent molecules form cross-links, holding collagen molecules together in parallel arrays.
How are collagen fibrils and fibres formed?
Collagen fibrils: Formed when multiple collagen molecules are arranged parallel to each other and stabilized by covalent cross-links.
The staggered arrangement of collagen molecules in fibrils creates a striated pattern visible under electron microscopes.
Collagen fibres: Created when many fibrils are packed together.
The fibres are aligned with the direction of forces they withstand, providing strength and stability.
How does collagen’s structure relate to its function?
Tensile Strength:
The triple helix structure, stabilized by numerous hydrogen bonds, allows collagen to withstand high pulling forces without stretching or breaking.
Staggered ends within fibrils provide additional strength.
Flexibility:
Its structure provides flexibility while maintaining rigidity, making it suitable for connective tissues.
Stability:
The high levels of proline and hydroxyproline result in R group repulsion, which stabilizes the structure further.
Insolubility:
The long length of collagen molecules and its tightly packed structure prevent it from dissolving in water, essential for structural roles.
How does collagen compare to haemoglobin?
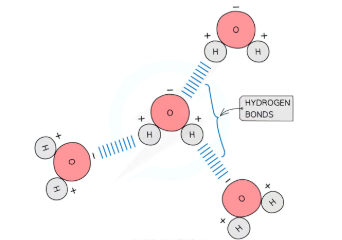
How does hydrogen bonding occur between water molecules?
Polarity: Water molecules are polar due to an uneven sharing of electrons in their covalent bonds.
Oxygen atom (δ⁻): Attracts electrons more strongly, making it weakly negative.
Hydrogen atoms (δ⁺): Become weakly positive.
Hydrogen Bonds: Form between the oxygen atom of one water molecule and the hydrogen atom of an adjacent molecule.
These bonds are weak individually but collectively form a strong and stable structure.
Constantly breaking and reforming, especially in liquid water.
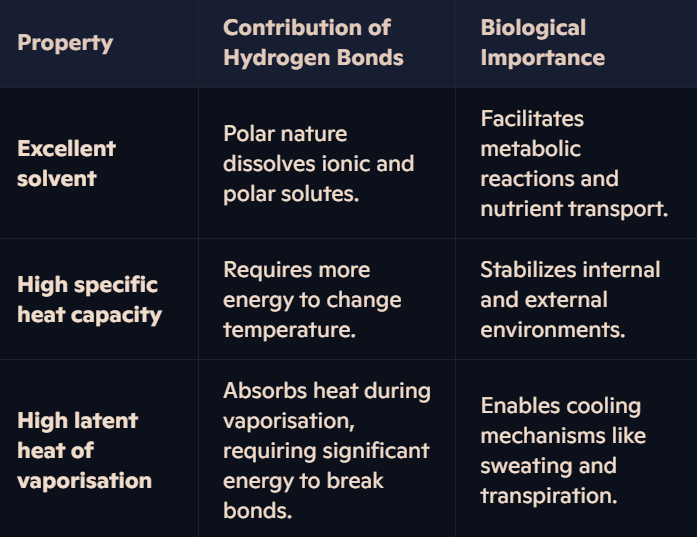
Why is water an excellent solvent, and how does this relate to hydrogen bonding?
Water’s polarity allows it to dissolve many substances:
Hydrophilic (polar) solutes: Interact with water molecules as the positive and negative ends of water attract ions and polar molecules.
Role in living organisms:
Acts as a medium for metabolic reactions in cells.
Enables the transport of nutrients, gases, and waste products in organisms.
Facilitates reactions by allowing ionic and polar compounds to dissolve.
How does water’s high specific heat capacity benefit living organisms?
Definition: Specific heat capacity is the amount of energy required to raise the temperature of 1g of water by 1°C.
Role of Hydrogen Bonds:
A large amount of energy is needed to break the extensive hydrogen bonds between water molecules.
This minimizes temperature fluctuations in water.
Biological Importance:
Maintains stable environments for aquatic organisms by moderating temperature changes.
Helps maintain a constant body temperature in organisms, vital for enzyme function.
How does water’s high latent heat of vaporisation benefit living organisms?
Definition: The latent heat of vaporisation is the energy required to change 1g of liquid water into vapour without changing its temperature.
Role of Hydrogen Bonds:
A significant amount of energy is needed to overcome hydrogen bonds during vaporisation.
Biological Importance:
Cooling Mechanism: Enables water to efficiently cool organisms through evaporation (e.g., sweating and transpiration).
Prevents dehydration by conserving water in the body while still regulating temperature.
How do hydrogen bonds contribute to the unique properties of water?