Diffusion of Respiratory Gases
1/54
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
55 Terms
Pulmonary Gas exchange and transport steps (6+photo)
O2 enters the blood at alveolar capillary interface
O2 transported in blood is dissolved in plasma or bound to haemoglobin inside RBCs
O2 diffuses into cells
CO2 diffuses out of cells
CO2 transported is dissolved / bound to hemoglobin / as HCO3-
CO2 enters alveoli at alveolar-capillary interface
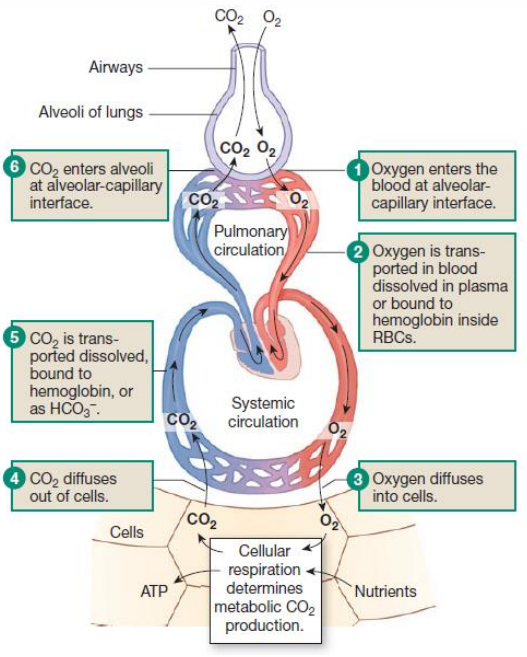
What does cellular respiration determines?
Metabolic CO2 production
Gas exchange in the lung definition
Occurs across a pressure gradient to reach an equilibrium, allowing O2 to enter the blood and CO2 to be expelled
Gas exchange in the lungs results in (3)
Increased O2 concentration in the blood
Diffusion of gases (O2 and CO2) across the alveolar and capillary membranes
Decreased CO2 concentration in the blood
Gas exchange is promoted by + reason (3+3)
Large surface area
due to numerous small alveoli (reduced by the destruction of alveolar walls in emphysema)
Thin single-cell layer
for efficient and quick diffusion, strengthened by collagen proteins for structural integrity
Elastin fibers
aid in the alveoli's ability to recoil after expansion
Pressure Gradient for Gas Exchange depends on
the partial pressure of the gas in alveolar air and plasma (dissolved gas)
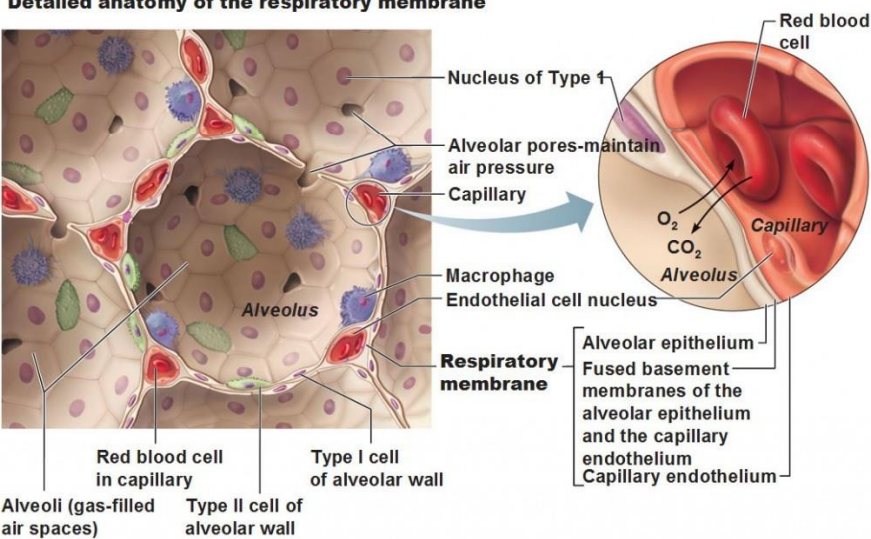
Structure of Alveoli - ANATOMY (2)
consists of clusters resembling a honeycomb at the ends of respiratory bronchioles
single outpouchings along the respiratory bronchioles
Alveoli main types of cells - HISTOLOGY (2)
Type I Alveolar Cells
Type II Alveolar Cells
Type I Alveolar Cells (2)
Make up 95-97% of lung cells
Responsible for gas exchange across a thin basement membrane
Type II Alveolar Cells (2)
Secrete surfactant to reduce surface tension
Reabsorb sodium (Na⁺) and water (H₂O) to prevent fluid build-up in the lungs
Detailed anatomy of respiratory membrane (photo)
Rate of Diffusion depends on with examples (4+4)
Surface area (alveoli)
Fx.: Emphysema (reduced surface area)
Barrier permeability (thin basement membrane)
Fx.: Fibrotic scars (decreased permeability)
Distance of Diffusion
Fx.: Pulmonary Oedema (increased diffusion distance)
Concentration gradient of gas
Fx.: COPD (decreased alveolar ventilation)
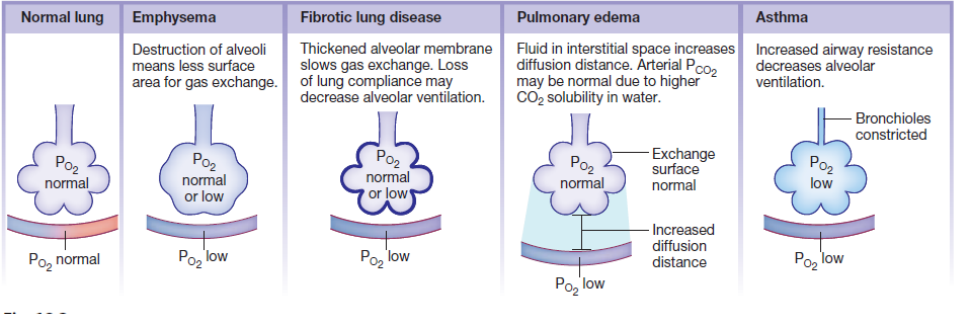
Factors/diseases influencing Diffusion of Gases (4+photo)
Emphysema
Fibrotic Lung Disease
Pulmonary oedema
Asthma
Maximum amount of gas dissolved in a fluid depends on (3)
Solubility of the gas in the fluid
Temperature of the fluid
Partial pressure of the gas
Henry's Law
Since solubility and temperature in blood are constant, the concentration of a gas in plasma depends on the partial pressure of the gas
Dalton’s Law
Total Pressure of a Gas Mixture = ∑ of the pressures of each gas in the mixture
Partial Pressure definition
the pressure that a particular gas in a mixture exerts independently
Partial Pressure Equation
Partial pressure = total pressure x fraction of that gas in the mixture
Change in Air Constituents as It Moves Into the Lungs (5)
External Air
Atmospheric Air
Air in Anatomical Dead Space
Inspired Air - in respiratory zone
Alveolar Air
External Air composition - Air Constituents
PN₂ + PO₂ + PCO₂
= Atmospheric Air
Atmospheric Air value - Air Constituents (2)
Pdry atmosphere = PN₂ + PO₂ + PCO₂ = 760 mmHg
PO₂ = 21% of 760 mmHg = 159 mmHg
Increase in Altitude of Atmospheric Air (3)
Atmospheric pressure decreases
PO₂ is reduced
can affect the oxygen available for gas exchange in the lungs
Air in Anatomical Dead Space - Air Constituents (4)
No gas exchange occurs (nose, mouth, larynx, trachea, bronchi, and bronchioles)
Composition: PN₂ + PO₂ + PCO₂ + P water vapor
Less PO₂ and more PCO₂ due to gas exchange at the alveoli
Conducting Zone
=inspired air
Inspired Air - Air Constituents (4)
Pwet atmosphere = PN₂ + PO₂ + PCO₂ + PH₂O
Pwater vapor at 37°C = 47 mmHg
The air becomes saturated with water vapor at body temperature (37°C), adding 47 mmHg of pressure to the total
PO₂(sea level) = 21% of (760 mmHg - 47 mmHg) = 150 mmHg
Alveolar Air equation - Air Constituents
PN₂ + PCO₂ (inc.) + PO₂ (dec.) + P water vapor + Temperature 37°C
Alveolar Air Composition (4)
Increase in PCO₂ due to gas exchange at the alveoli
PO₂ decreases
Temperature is constant at 37°C
Saturated with water vapor (100% humidity)
PO₂ is diminished to about 105 mmHg at sea level due to gas exchange in the alveoli
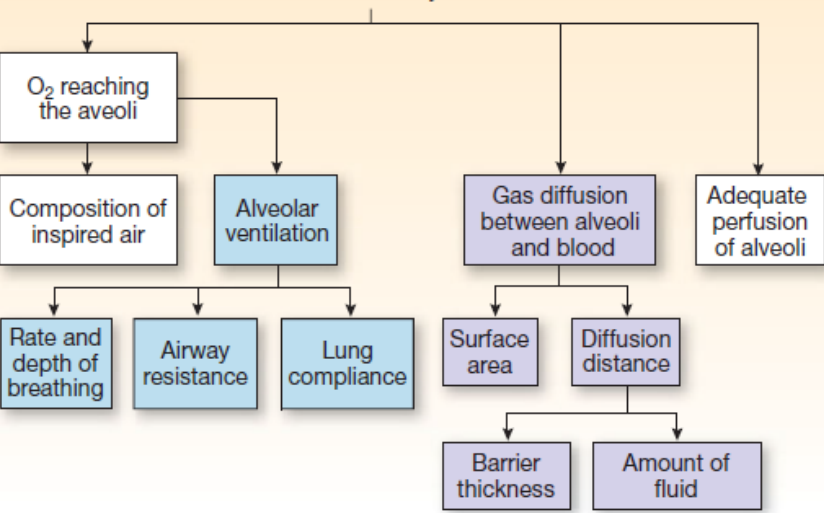
Alveolar Gas Exchange influenced by (12-photo)
Oxygen Electrode (4)
produces an electric current in proportion to the concentration of dissolved oxygen in the plasma
not measure oxygen bound to haemoglobin
Useful for assessing lung function (gas exchange)
PO₂ in systemic blood is typically 5 mmHg lower than in alveolar air (105 mmHg at sea level)
Oxygen Content in Blood (5)
At normal PO₂ (100 mmHg), whole blood contains almost 20 mL of O₂ per 100 mL of blood.
Plasma contains 0.3 mL of O₂.
Red Blood Cells (as oxyhemoglobin) contain 19.7 mL of O₂.
Breathing 100% oxygen increases the amount of oxygen dissolved in the plasma
not significantly change the total oxygen content in the blood
Oxygen Diffusion to Tissues (2)
Increasing plasma PO₂ concentration at normal PO₂ → increases the rate of oxygen diffusion to tissues
Oxygen bound to haemoglobin in RBCs must first dissolve in plasma before diffusing into cells
Pulse Oximeter (6)
measures oxyhemoglobin saturation
Non-invasive method
Clipped to the ear pinna or finger
Works using two LED lights (red and infrared).
Oxyhemoglobin and deoxyhemoglobin absorb light differently
Sensors and microprocessors determine the percentage of oxyhemoglobin in the blood
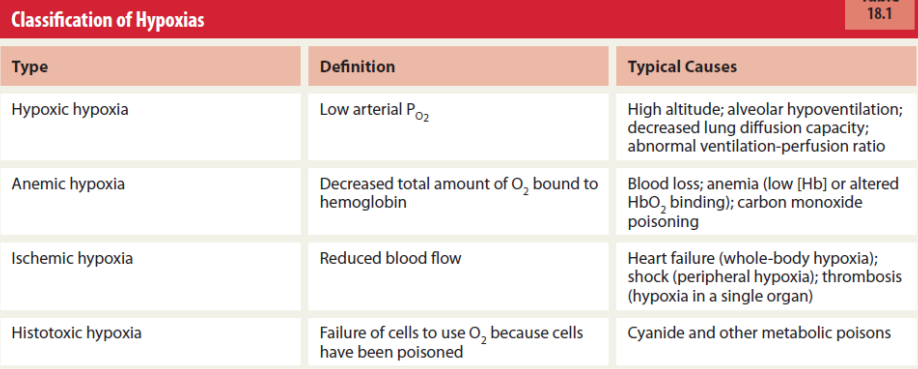
Classification of Hypoxia + causes (photo)
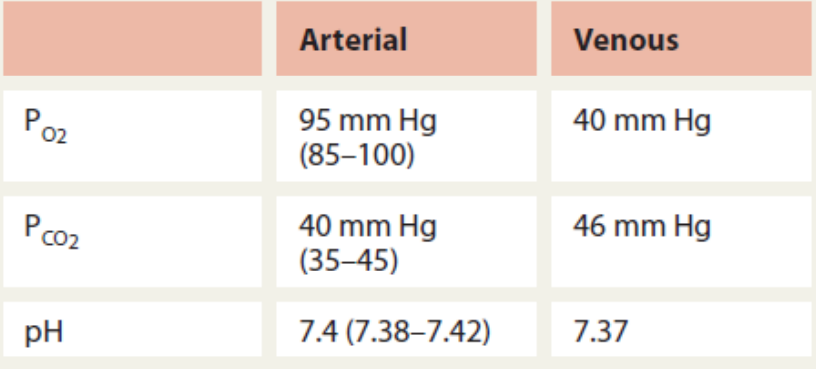
Normal Blood Values in Pulmonary Medicine (photo)
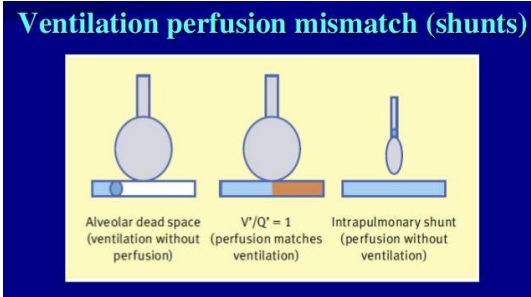
Ventilation-Perfusion Mismatch Types (2)
Dead Space Ventilation
Shunt
Due to high altitude
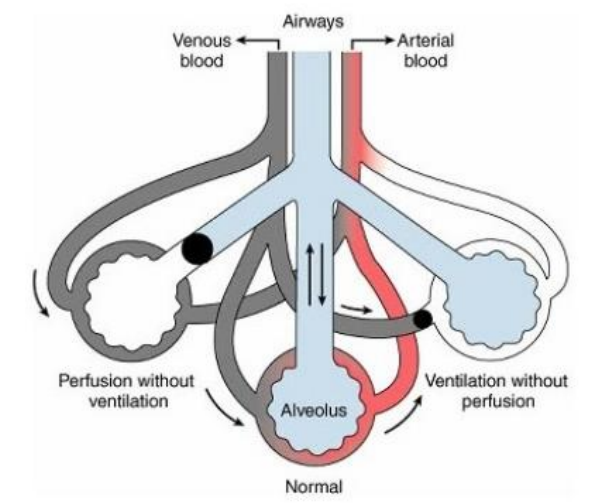
Dead Space Ventilation (5)
Left
Good ventilation
No CO2 reaches
No perfusion (blood flow) to the alveoli
V/Q > 1
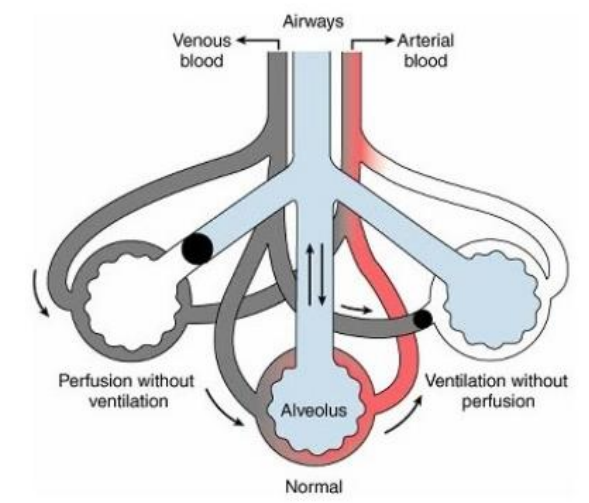
Shunt (5)
Right
Bronchus is blocked
No airflow reaching the alveoli
low PO2
No CO2 is exchanged
V/Q = 0
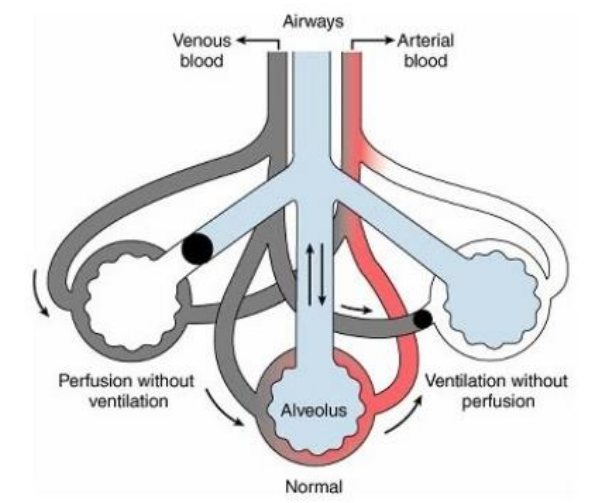
Ventilation/Perfusion Ratio (V/Q Ratio)
V (Volume of Air ventilated, L/min) / Q (Volume of Blood flow, L/min)
During standing (5)
Blood flow to the base of the lungs ↑ due to gravity
At the apex of the lungs, the intrapleural pressure is lower - fluid
keeping alveoli more open but less compliant
resulting in less efficient ventilation (smaller volumes of air exchanged)
If this effect is not in the same proportion → leading to overventilation (underperfusion) at the apex
V/Q mismatch
occurs when there is a disruption in ventilation, gaseous exchange, or blood circulation
Conditions that cause V/Q mismatch and it’s effect on V/Q (3+3)
Pneumonia
V/Q: Decreases
Pulmonary embolism
V/Q increases
Pulmonary oedema
V/Q: Decreases
Pneumonia causes
Decreased ventilation & gaseous exchange due to inflammation and mucus
Pulmonary embolism causes
Disrupted blood circulation due to a blockage
Pulmonary oedema causes
Decreased gaseous exchange due to fluid in the lungs
Pulmonary Circulation (5)
Pulmonary arterioles constrict when PO₂ is low and dilate when PO₂ is high
This response is opposite to that of systemic circulation
The purpose is to match ventilation to perfusion:
Blood flow is reduced in areas with low ventilation (low PO₂)
Blood flow is increased in well-ventilated alveoli
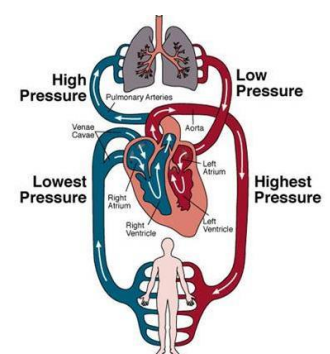
Pulmonary Circulation in Adult (4)
Low pressure (10 mmHg) compared to systemic circulation (100 mmHg)
Low vascular resistance helps protect against pulmonary oedema by maintaining lower filtration pressure
Left ventricular heart failure→ lead to pulmonary hypertension
increasing filtration pressure and potentially causing pulmonary oedema
Pulmonary Circulation in the Fetus (6)
Lungs are partially collapsed - not involved in ventilation
High vascular resistance in the fetal lungs
not yet functioning for gas exchange
Blood shunting occurs:
From the right atrium to the left atrium through the Foramen Ovale
From the pulmonary artery to the aorta through the Ductus Arteriosus
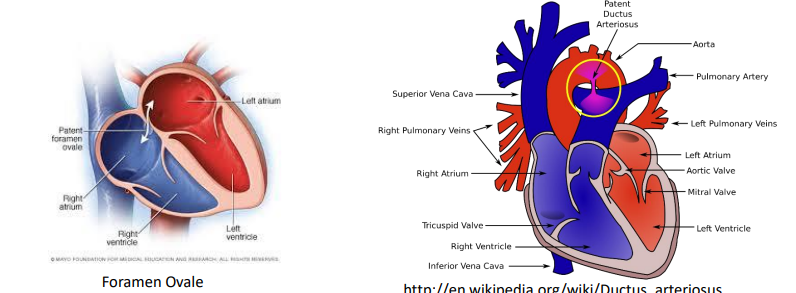
Blood shunts function
These shunts help bypass the non-functional lungs and direct blood to the rest of the body, allowing fetal circulation to operate without relying on the lungs.
Pulmonary Circulation After Birth (6)
Vascular resistance falls due to:
Physical stretching of the lungs during inspiration
Dilatation of pulmonary arterioles in response to increased alveolar PO₂
Blood flow through pulmonary vessels increases sharply as a result
Foramen ovale and ductus arteriosus close
redirecting blood flow through the lungs for oxygenation
Hyperbaric Oxygen Therapy (Hyperbaric Unit) (2)
100% oxygen is administered at 2-3 atmospheres pressure
Discontinued in premature infants due to the risk of fibrotic deterioration of the retina, which can lead to blindness
Hyperbaric Oxygen Therapy used to treat (3)
Decompression sickness
Severe traumatic injury (e.g., crush injury)
Very poor peripheral circulation
Conditions caused by High Partial Pressures of Gases (3)
Oxygen Toxicity
Decompression Sickness (the Bends)
Nitrogen Narcosis
Oxygen Toxicity (6)
Breathing 100% oxygen at 2-3 atmospheres pressure
can be safely tolerated for a few hours
Higher partial pressures of oxygen can cause:
Oxidation of enzymes
Damage to the nervous system → coma and death
Divers use gas mixtures with inert gases
(e.g., nitrogen, helium) to avoid oxygen toxicity
Decompression Sickness (the Bends) (7)
Nitrogen dissolves in plasma as pressure ↑
Rapid ascent (after diving or flying) causes dissolved nitrogen to form gas bubbles
can block small blood vessels
This blockage leads to:
Muscle and joint pain
Other damage from obstructed blood flow
Also occur if an airplane descends too rapidly from high altitudes (even with a pressurized cabin)
Nitrogen Narcosis (5)
Nitrogen is physiologically inert - becomes harmful under high pressures
Large amounts of dissolved nitrogen can cause symptoms similar to alcohol intoxication:
dizziness, extreme drowsiness, and a condition known as "rapture of the deep"
Symptoms develop after prolonged exposure (more than an hour)
nitrogen takes time to dissolve into the body
Breathing 100% Oxygen (3)
Reduces body's response to PCO2
Chemoreceptor sensitivity is blunted
Ventilation rate decreases