Chapter 22 Materials
1/102
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
103 Terms
phase diagrams and phase transformations
central to understanding microstructure, properties, and processing paths.
metallic alloy
a mixture of a metal with other metals or non-metals (often form precipitates/inclusions).
Components
the chemical elements that make up alloys.
binary alloy
contains two components.
Alloy compositions
defined by concentration in weight % or atom %.
phase
A region of a material that is structurally homogeneous (same crystal structure) and distinct (interface with surroundings)
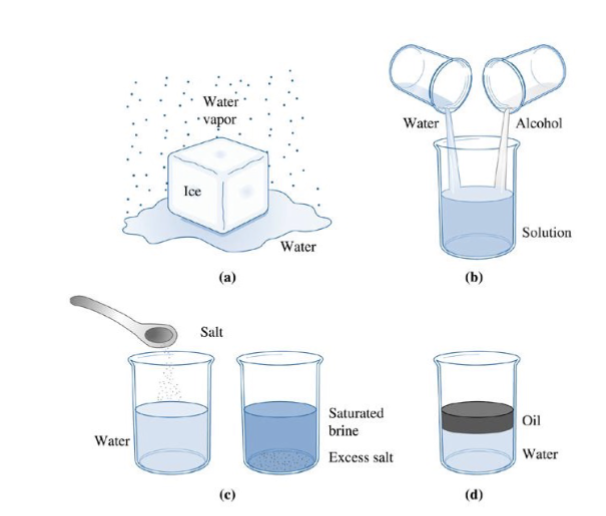
Three forms of water (solid, liquid gas) are each one; water and alcohol have unlimited solubility (one); salt + water have limited solubility (excess salt means 2); oil and water have virtually no solubility (2)
constitution of an alloy
described by:
• The identity of phases present. (at equilibrium)
• The composition of each phase.
•The fraction of each phase
these properties are represented in a phase diagram
stable
At thermodynamic equilibrium, the constitution is _______ (no change is possible).
equilibrium constitution
the state with the lowest Gibbs free energy for a given composition, temperature, and pressure; shown in phase diagram; minimizes energy state
phase diagram
a diagram with temperature and composition as axes, showing the equilibrium constitution. It is like a map and is divided into fields or regions.
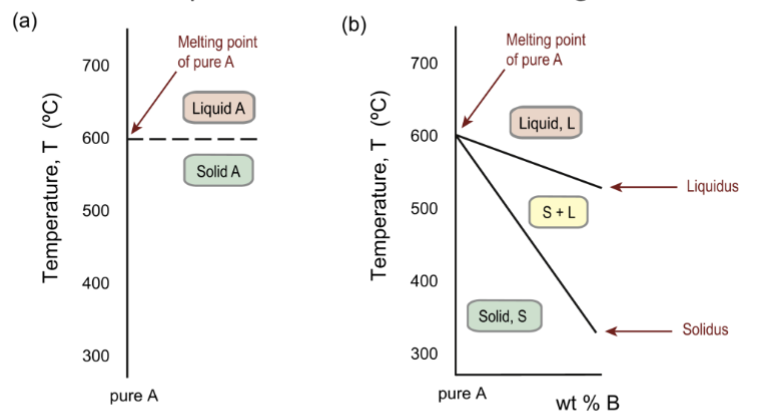
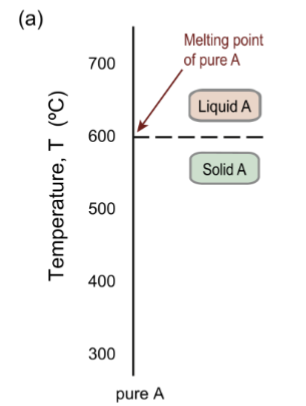
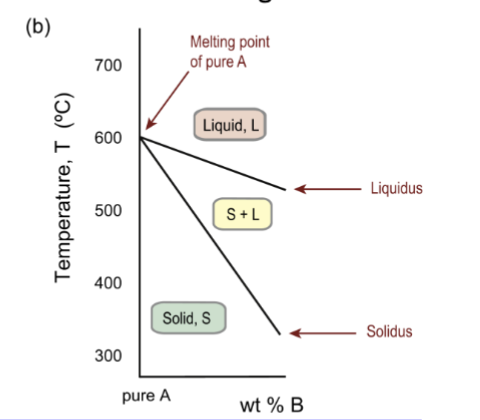
one dimensional phase diagram
for a pure substance, a temperature scale showing the phase boundary between solid and liquid – the melting point
two elements in phase diagram
the A-rich end of a binary A–B phase diagram, illustrating partition of the melting point between solidus and liquidus boundaries
solid is always crystalline; S+L is the freezing range, and both solid and liquid are present
Isomorphous Phase Diagram
simplest type of phase diagram
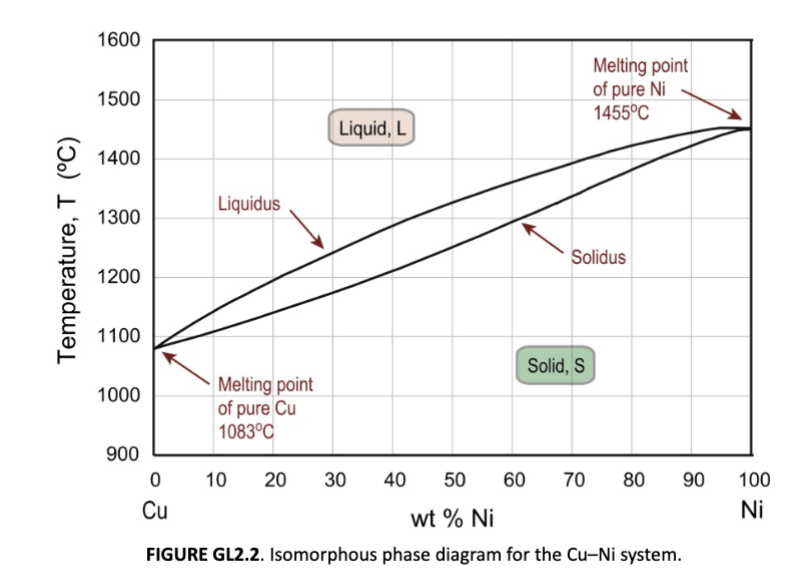
isomorphous
For two components, we have a binary phase diagram. If there is complete solubility in the solid phase, the system is said to be…
liquidus line
boundary between liquid and L+S two-phase field
Solidus line
boundary between solid and L+S two-phase field.
Freezing range
Difference between solidus and liquidus temperatures for a given composition.
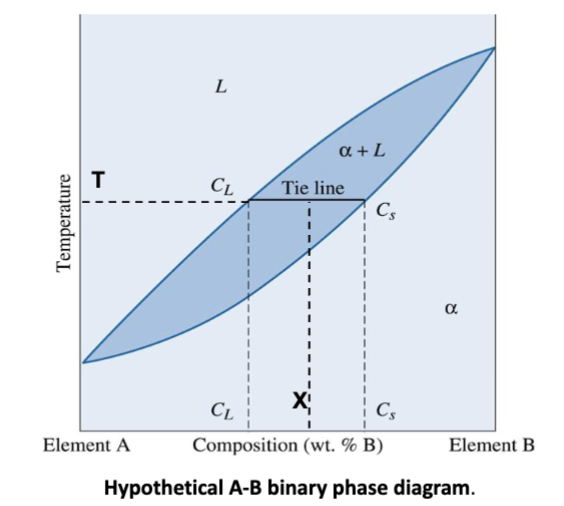
tie line
For an alloy at coordinates T-X inside a two-phase region, a _______ at the temperature of interest fixes the compositions of the two phases.
Constant temperature → extend line to liquidus and solidus to get composition of liquid and solid
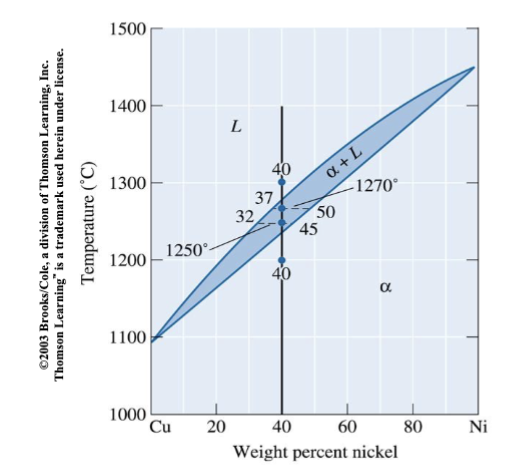
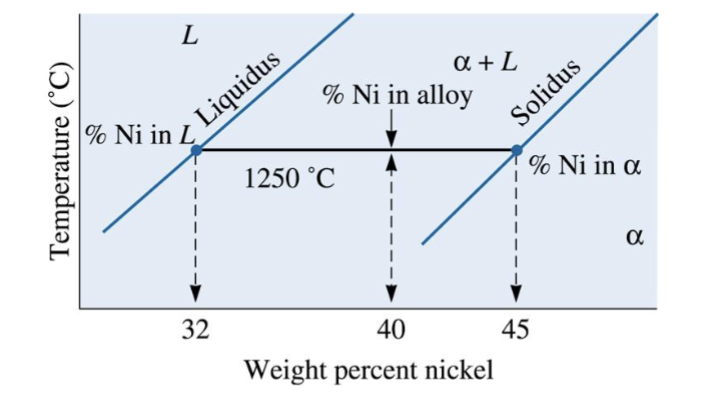
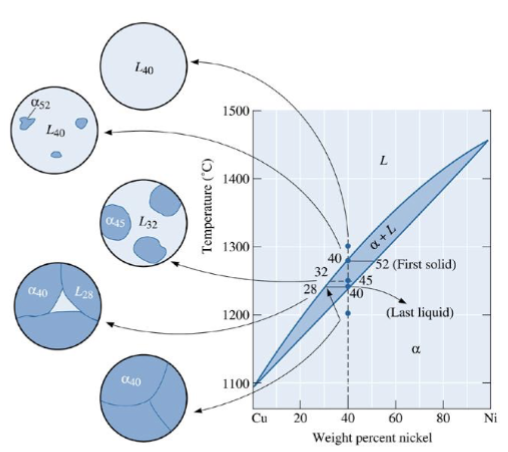
composition of phases in Cu-40% NI alloy at different temperatures
The vertical line represents overall composition of the alloy:
1300°C: Only liquid is present. The liquid must contain 40%Ni, the overall composition of the alloy.
1270°C: Two phases are present. The liquid contains 37%Ni, and the solid contains 50%Ni.
1250°C: Again, two phases are present. The tie-line drawn at this temperature shows that the liquid contains 32%Ni and the solid contains 45%Ni.
1200°C: Only solid is present, so the solid must contain 40%Ni
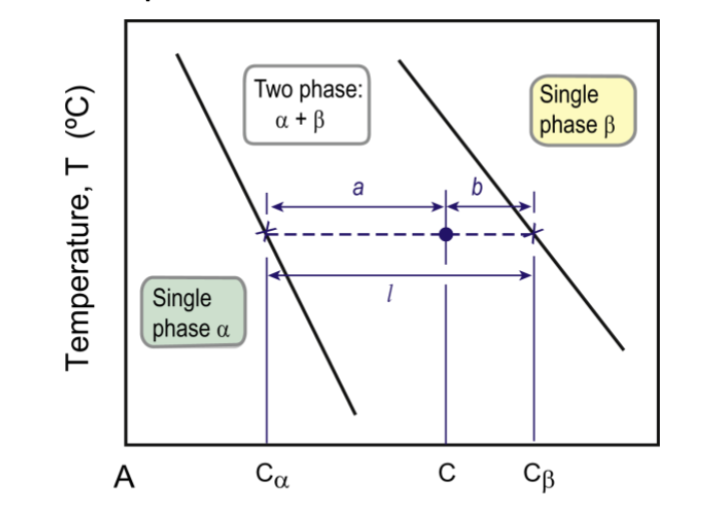
phase fraction
=(opposite arm of lever)/(total length of tie-line); part of the lever rule for proportion of phases
fraction α = (C-C1)/(C2-C1)
fraction L = (C2-C)/(C2-C1)
lever rule for two solids composition
fraction α = (b/l)
fraction β = (a/l)
solvus line
the boundary between the single- and two-phase regions; saturation level for solutions increases with temperature
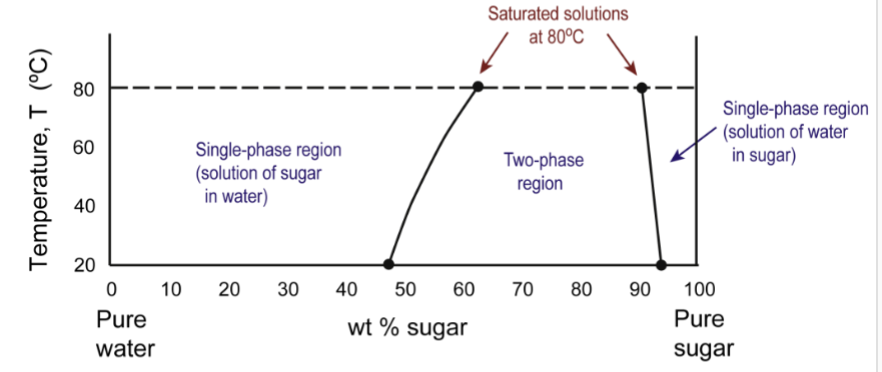
solubility
how much can we add of one material to another without producing an additional phase
unlimited solubility
soluble in all proportions; water and alcohol as liquid, Ni and Cu in both liquid and solid. In the solid (a solid solution), both Ni and Cu are FCC elements.
limited solubility
soluble up to a point where second phase begins to form (water and salt, Cu and Zn). Can also mean almost no solubility (oil and water, Cu and Pb)
Hume-Rothery Rules
necessary but not sufficient conditions for unlimited solid solubility:
size - within 15% difference in atomic radius
crystal structure - same for both elements
valence - same for both types of atoms
electronegativity - similar for both types of atoms
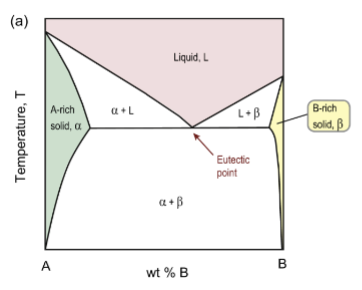
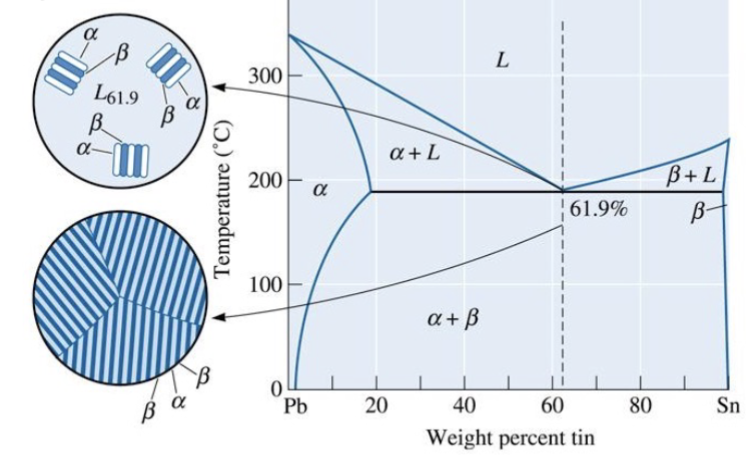
eutectic point
The lower limit of the single-phase liquid field formed by the two liquidus boundaries; example of an invariant point
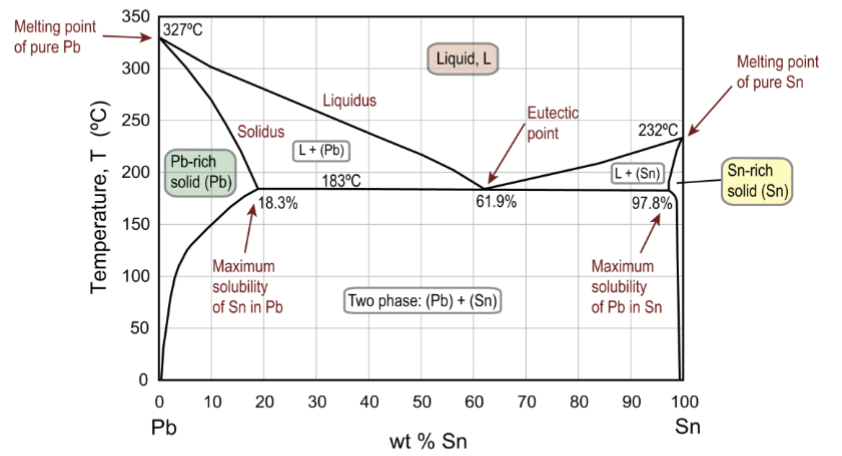
eutectic reaction
at this point, this reaction occurs on cooling: Liquid → Pb-rich solid solution + Sn-rich solid solution (essentially two different solids upon cooling)
maximum solubilities
in the solid phases, usually occur at the same temperature
intermediate phases
many systems exhibit these. These compounds often have high melting points and are very stable. Ex: intermetallic compounds, where metal atoms are preferentially bonded to the other type of atom
Pretty strong and low ductility
intermetallic compounds.
If the components are both metals with stoichiometric or near-stoichiometric compositions (in atomic percent), then we call them this.
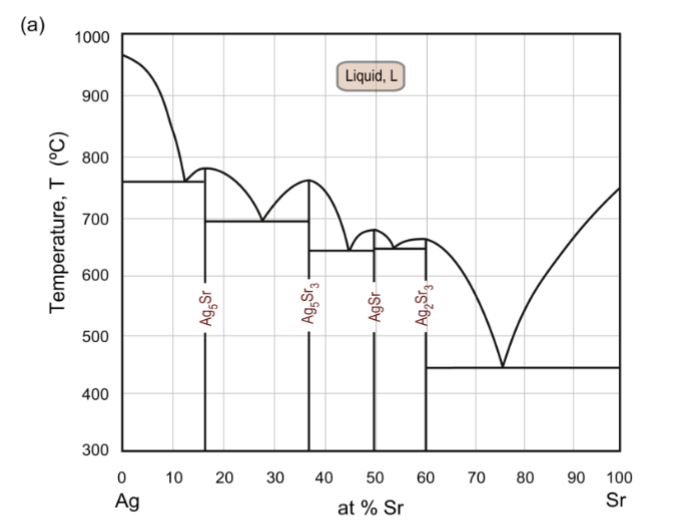
intermediate phase diagram
each intermediate phase has its own eutectic point/reaction. Straight lines mean fixed stoichiometry. This one has four distinct intermediate phases/compounds
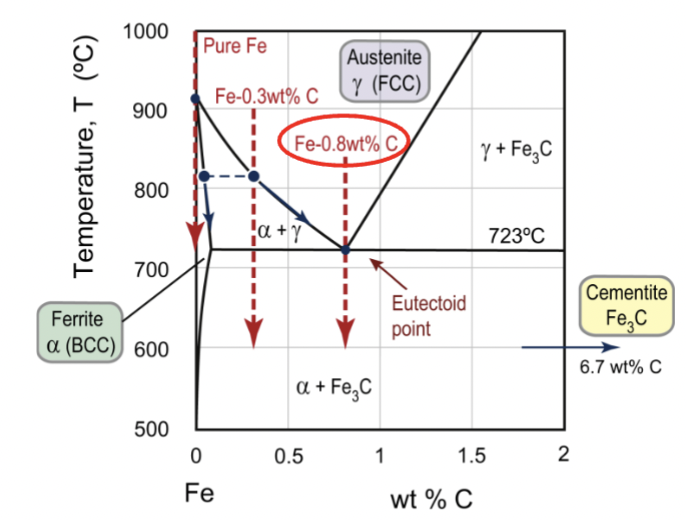
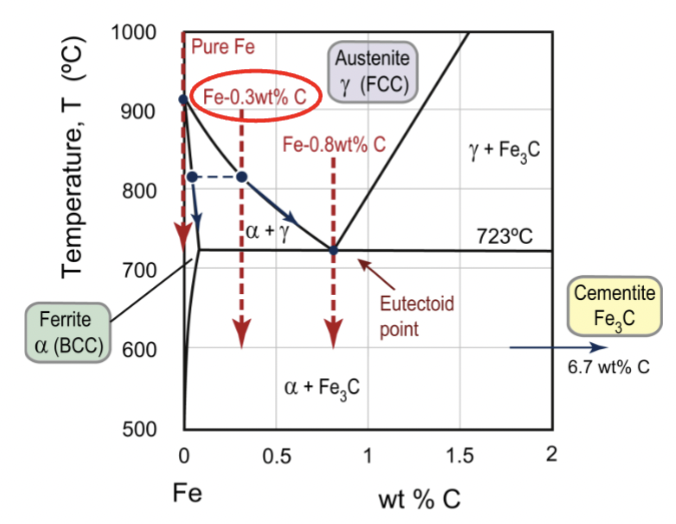
Fe-C compounds
encompasses cast irons (high wt% C) and steels (lower wt% C)
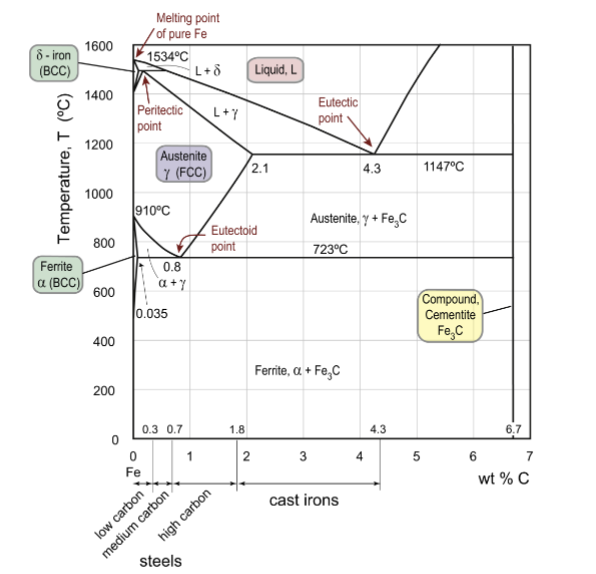
austenite
FCC iron phase, gamma; at elevated temperatures, there is a big solubility of carbon compared to BCC (wide range of properties)
up to 2.1wt% C in solid solution
ferrite
BCC iron phase, can be denoted alpha (low) or delta (high) based on temperature; unstable intermediate; same phase but just different temperatures
α: up to 0.03wt% C in solid solution
δ: up to 0.08wt% C in solid solution
cementite
Fe3C, iron carbide; about 25% atomic carbon; up to 6.7wt% C
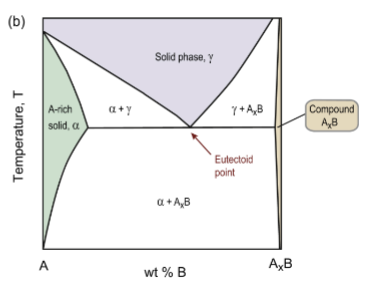
eutectoid point for Fe-C
the lower limit of a single-phase solid field formed by two falling phase boundaries intersecting in a ‘V’. Reaction is γ → α + Fe3C.
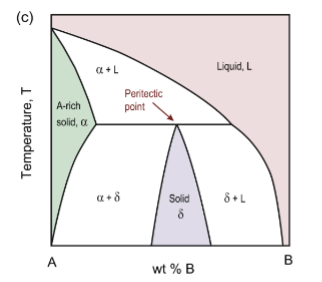
peritectic point for Fe-C
reaction is L + δ → γ upon cooling
four basic types of reactions
eutectic, eutectoid, peritectic, and peritectoid
eutectic reaction
liquid → two different solids upon cooling
L → α + β
eutectoid reaction
solid → two other, different solids upon cooling
γ → α + AxB
peritectic reaction
solid + liquid → other solid upon cooling (2 phases to get 1 phase)
α + L → δ
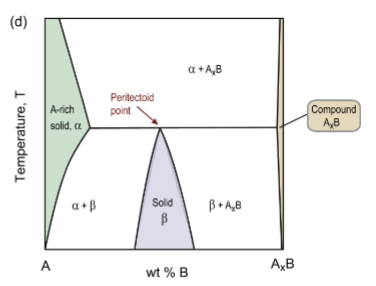
peritectoid reaction
solid + solid → other solid upon cooling
α + AxB → β
phase transformations
driven by reduction in free energy (the “driving force”, high → low is spontaneous); usually involve diffusion; occur by a two-stage process: nucleation and growth
time temperature transition diagrams
capture the extent of isothermal transformation as a function of time and temperature
nucleation and growth
for example, in a eutectic reaction, the solid phases first have to appear/form and then the first stage of forming the initial crystal is this first step; one the microcrystal exists, it grows (step 2)
nucleation
of a crystalline solid in a liquid, atoms in a pure metal liquid; small crystal of atoms forms, once this tiny crystal forms, then it grows.
the initial process that occurs in the formation of a crystal from a solution, a liquid, or a vapour, in which a small number of ions, atoms, or molecules become arranged in a pattern characteristic of a crystalline solid, forming a site upon which additional particles are deposited as the crystal grows
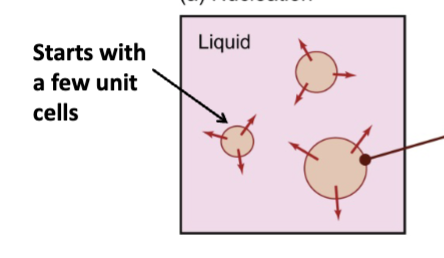
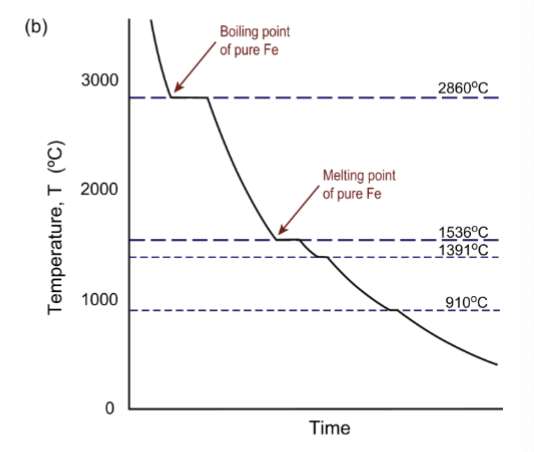
thermal arrests
occur when you remove heat in order for transformation to proceed; a large amount of heat has to be removed for the heat of vaporization or heat of fusion, so the material stays at that temperature for a while before condensing, melting, etc
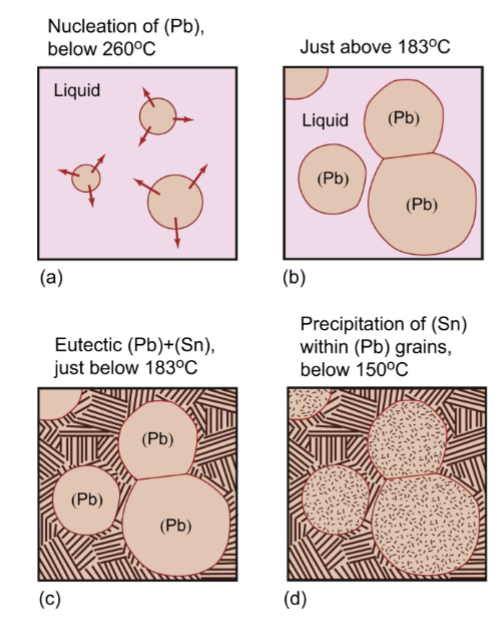
alloy cooling curve
solidify over a range of temperatures, and the plateau is replaced by a decreased cooling rate as the material gradually solidifies as temperature decreases.
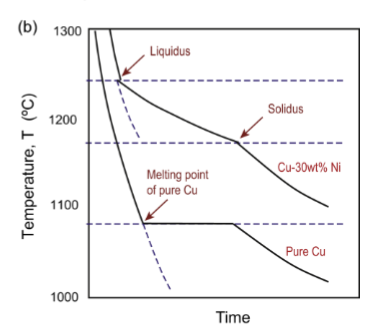
no thermal arrests in alloys
because only a tiny bit of solidification occurs at each temperature and it occurs over a range of temperatures. cools more slowly due to two types of heat removal for two materials.
change of slope
in alloy cooling curve, corresponds to liquidus/solidus temperatures
solid-liquid interface
boundary where a solid and liquid interact, with a perturbation of the liquid structure near the solid surface
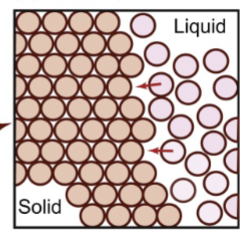
impingement
growth of nuclei and onset of this;
atoms cannot be perfectly in both crystals at the same time, leading to a disordered region or grain boundary
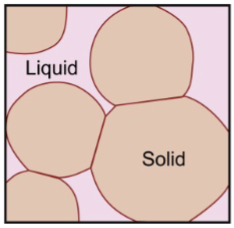
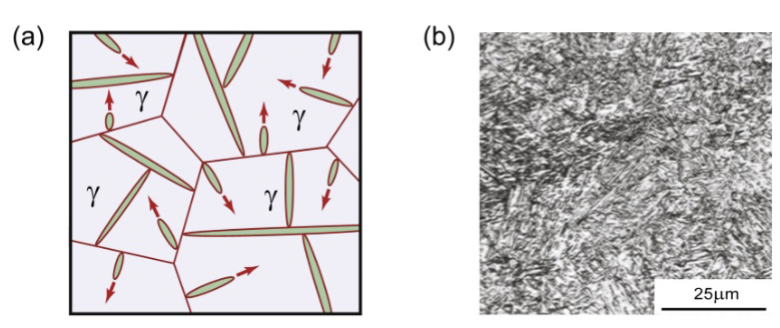
solidification of solid solution alloy
Example: Cu-40%Ni.
First solid to form: 52%Ni.
Diffusion is required so that the compositions of liquid and solid follow the liquidus and solidus curves.
Last liquid to freeze: 28%Ni.
composition changes: 52% → gives up Ni to liquid → goes down until it reaches 40% Ni
very slow cooling
in a solid solution alloy, you only can get a uniform equilibrium structure with …
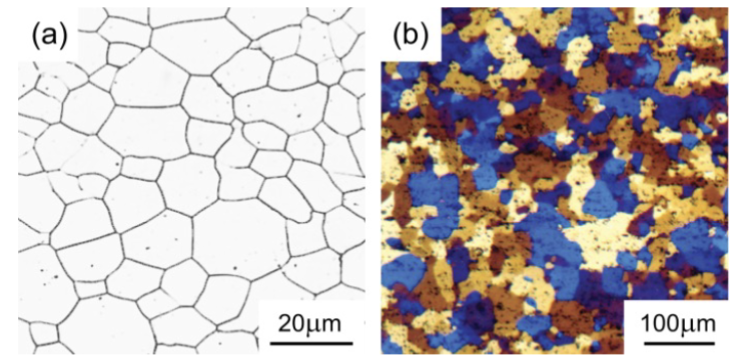
optical micrographs
(a) pure Fe and (b) pure Al. In (b) the etching technique produces color contrast between different crystal orientations – but it is still all one phase.
colors present different grains and crystal orientations
bonds
with the disorder on grain boundaries upon cooling a solid solution alloy, the _______ are not as strong on the edges as they are in the middle
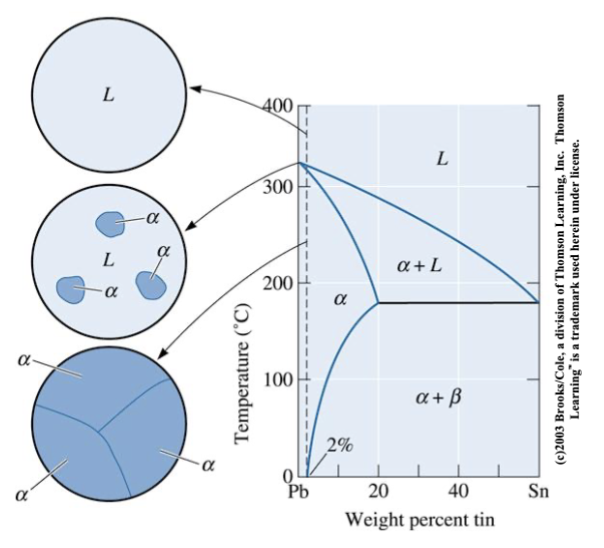
solidification with limited solubility
microstructure of a Pb-2%Sn alloy. The alloy is a single-phase solid solution
Composition Pb-2wt%Sn is special because it is the maximum solubility at room temperature; on the left side of this line, only alpha grains.
solid solution and precipitation hardening
kinds of strengthening mechanisms that could account for this alloy A composition
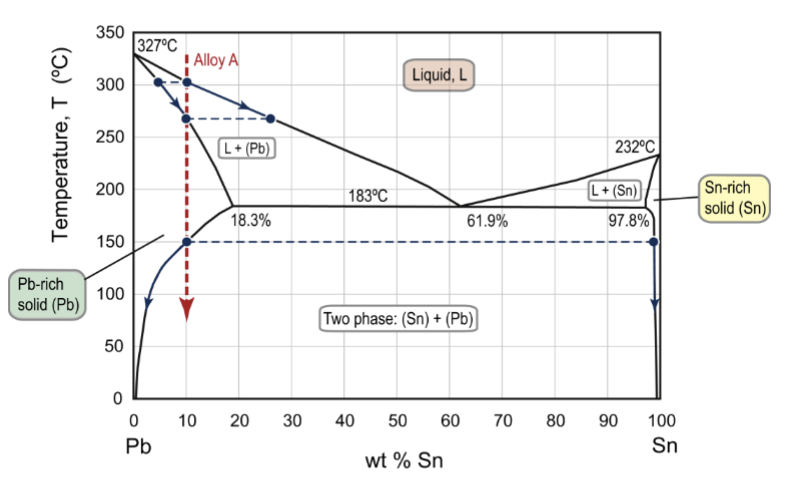
precipitation
of (Sn) within (Pb) grains, very fine little dots/rods → good strengthening
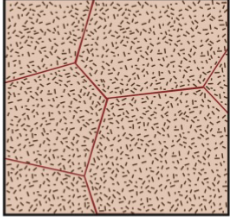
alloy cooled at eutectic point
the microstructure contains colonies of eutectic microconstituent instead of grains. Series of parallel layers that form colonies. Each layer is a lamella. 2D layers
proeutectic phase
phase that forms (on cooling) before the eutectoid austenite decomposes. It has a parallel with primary solids in that it is the first phase to solidify out of the austenite phase
Eutectic-like cooling but below the eutectic composition.
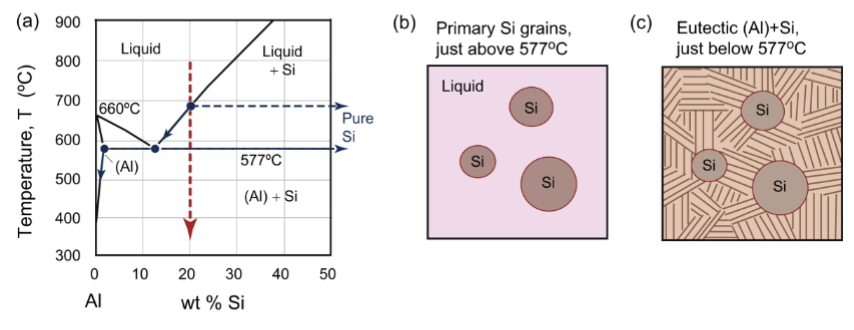
hypereutectic composition
those with a higher composition of species β and a lower composition of species α than the eutectic composition
Eutectic-like reaction but above the eutectic composition
kinetics
for solid-solid phase transformations, this is very important. Science of the rate at which reactions can occur — how long does something take to tranform
α → α + β (precipitation)
γ → α + β (eutectoid)
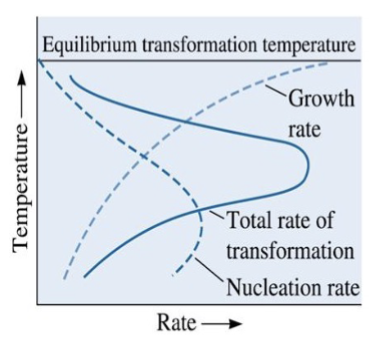
nucleation rate
is low small undercooling (ΔT) and high for large undercooling. Increases with increasing ΔT. Occurs most easily at interfaces (disorders in material that can accommodate changes)
growth rate
high for low undercooling and low for large undercooling (requires diffusion: e-Q/RT)
generally by long-range diffusion. Rate increases with increasing temperature
undercooling
difference between equilibrium temperature and actual temperature for the reaction to proceed; actual transformation occurs at temperature far from equilibrium (lower temp)
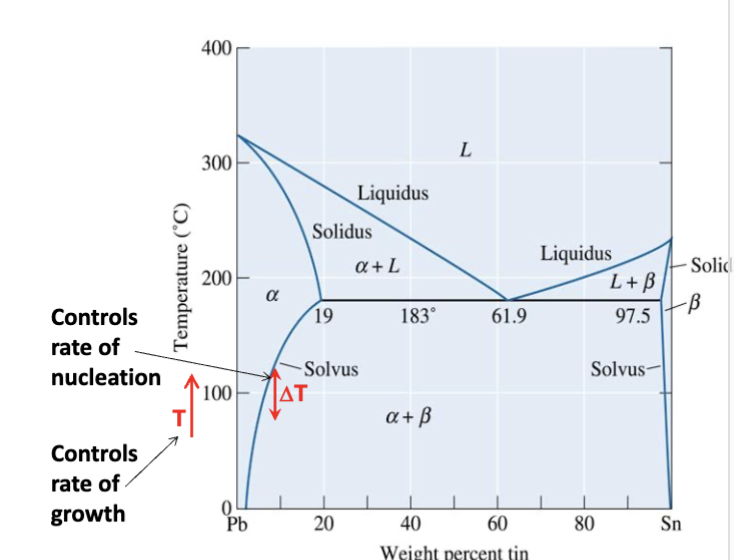
overall rate
of transformation depends on both nucleation and growth because you need nucleation in order for the reaction to begin. Growth determines the rest of the transformation.
temperature dependent
Both nucleation (N) and growth (G) are …….
N is low for small undercooling and high for large undercooling.
G is high for small undercooling and low for large undercooling (diffusion). Growth rate = A exp[-Q/(RT)]; Q is activation energy.
product of N and G rates
Overall transformation rate is a ______________. The transformation rate is usually a maximum at some intermediate temperature.
Close to equilibrium temperature → slow transformation
Start to deviate from equilibrium → larger rate
Even further from equilibrium → start to slow down again
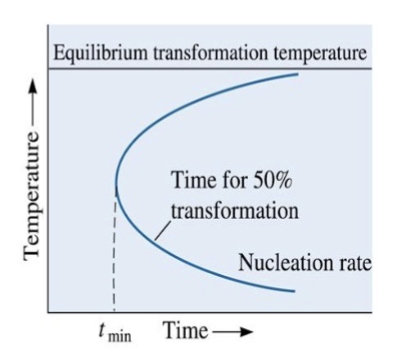
C curve
Time required for transformation is inversely related to rate of transformation. This produces a _______ in a TTT diagram
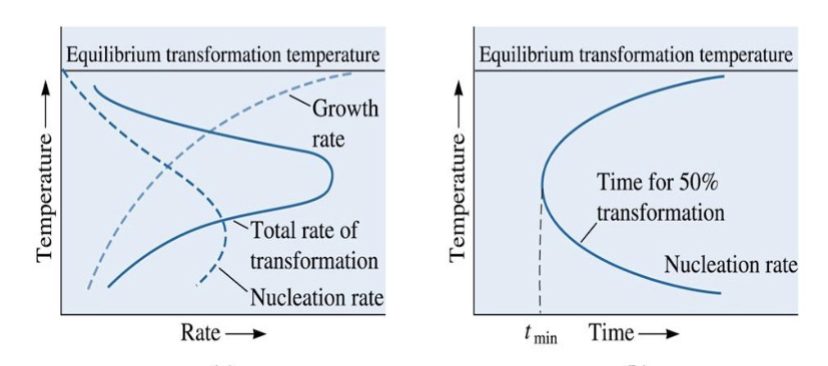
maximum transformation rate
Effect of temperature on rate of a phase transformation is the product of growth rate and nucleation rate contributions, giving a ______________ at a critical temperature
minimum time (tmin)
there is a ______ required for transformation, given by the “C-curve”
precipitation reactions
When we cross from Al solid solution to the two-phase region, the solubility of Cu in Al decreases and CuAl2 begins to form in the pre-existing grain structure.
Further from the solvus boundary, the nucleation rate increases
Compositional range for CuAl2 in (Al): between 0wt% CU and max solubility of copper in aluminum (5-6%)
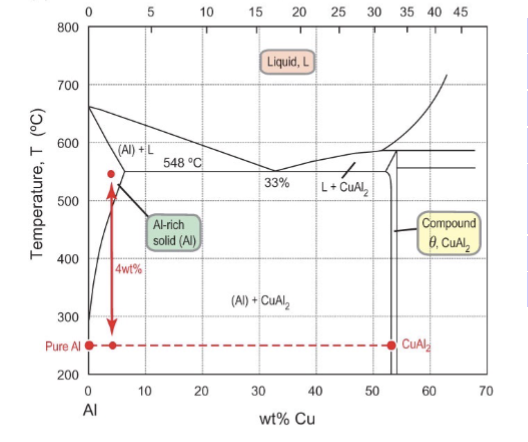
precipitates of CuAl2 in (Al)
cool slowly.
initially, CuAl2 precipitates tend to form on grain boundaries first (from there more rapidly). Diffusion is faster along grain boundaries leading to larger precipitates.
In the bulk of the grains, there is a much lower diffusion rate and nucleation occurs later because there is a barrier towards formation more inside the grains than elsewhere. Nucleation rate will be high and the growth rate slow, leading to widely dispersed, very small precipitates
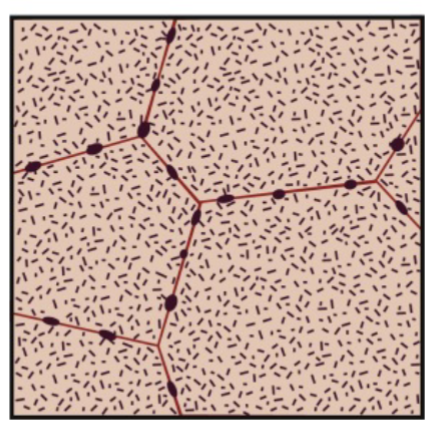
low ΔT and slow cooling
slow nucleation, fast growth = slow overall reaction. Probably heterogenous → form on defects, boundaries, surfaces, etc.
A few nuclei grow large (widely spaced large precipitates)
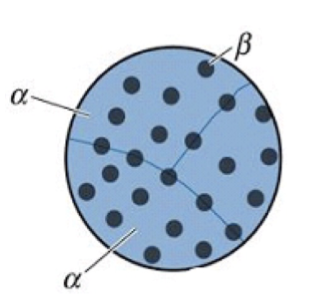
high ΔT and faster cooling
fast nucleation, slow growth = slow overall reaction. Probably homogeneous → not enough places for heterogeneous nucleation to occur.
Many nuclei that grow slowly (closely spaced fine precipitates). Good precipitation hardening.
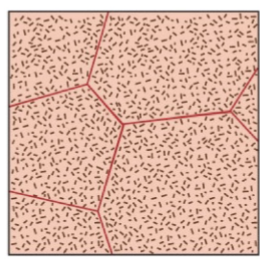
very rapid cooling
supersaturated solid solution. No precipitation occurs, even though there is a high driving force, nucleating and growth don’t have a chance to get started. Don’t see a transformation occurring
age or precipitation hardening
produces a uniform dispersion of fine, hard precipitates in a softer, more ductile matrix. Three steps: solution anneal, quenching, and aging
Extremely slow process at room temperature but allows the strength and ductility to be retained for practical use
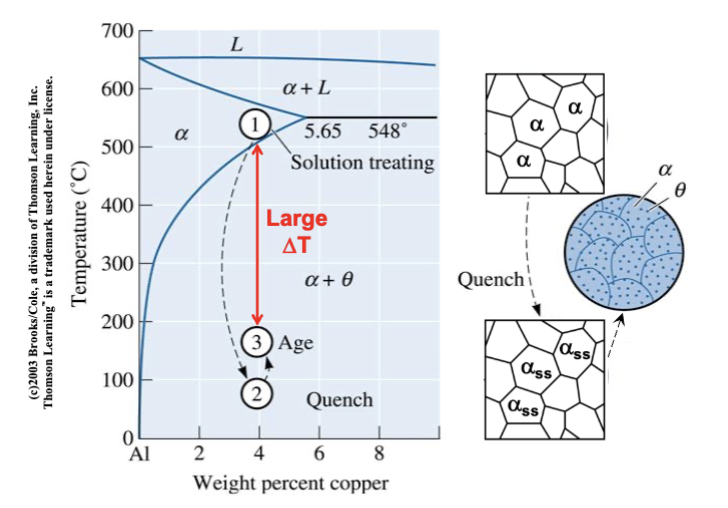
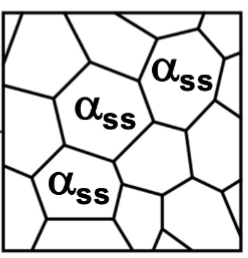
solution anneal
step one of age/precipitation hardening: goal is to dissolve any existing second phase by heating above the solvus boundary and entering a single phase region where all of the 2nd element is soluble in the Al-rich phase.
Homogenize the solid solution → allow diffusion to make composition uniform
quenching
step two of age/precipitation hardening: Rapidly cool to retain supersaturated solid solution (not at equilibrium). Prevent precipitation (no nucleation or growth)
aging
step three of age/precipitation hardening: heat supersaturated solution below the solvus boundary and precipitates nucleate and grow → get very fine dispersion → much higher strength → extremely fine spacing and very small precipitates
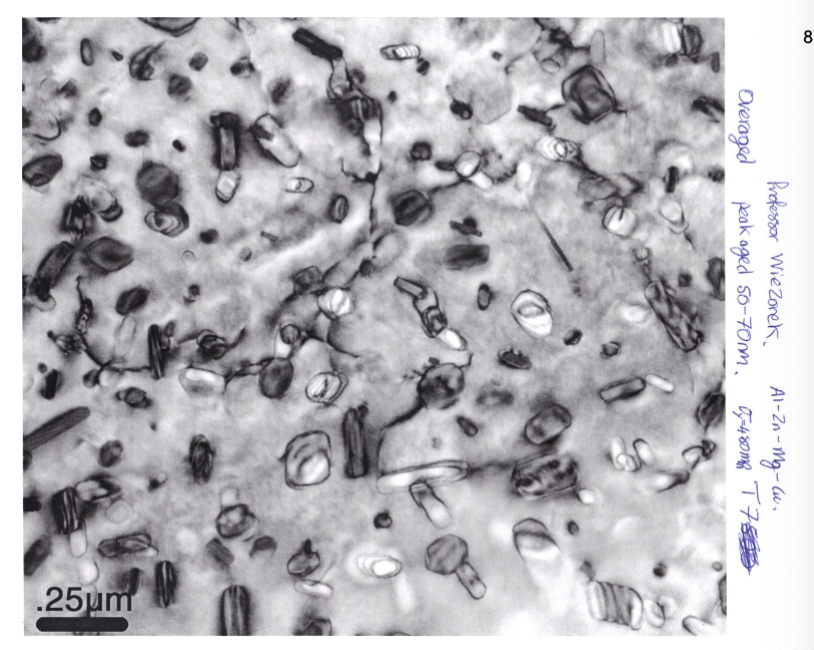
overaged
heated longer or at a higher temperature than to get peak strength (leading to larger particles). slightly weaker than the peak aging, but still with extremely small spacing.
phase transformation in carbon steels
includes eutectoid point, where austenite transforms to ferrite and iron carbide
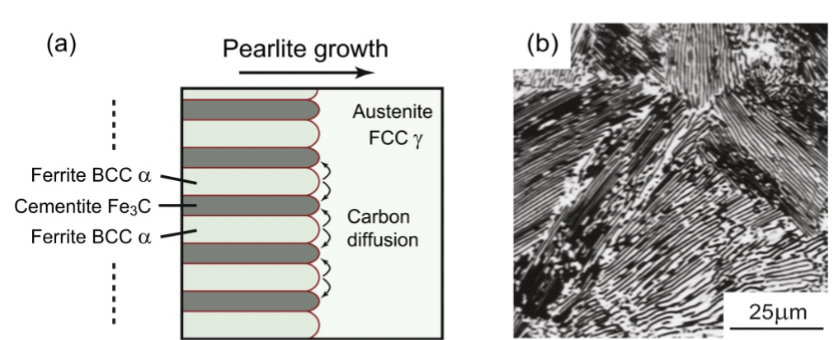
pearlite
carbon must diffuse to the growing cementite phase as the two-phase _______ grows (α-Fe is almost pure Fe)
a two-phase lamellar (parallel layers) mixture of ferrite and cementite. Eutectoid transformation. Not on phase diagram → α + Fe3C but can have different morphology.
low undercooling and slow cooling
Slow nucleation, fast growth = slow overall reaction.
A few nuclei grow large (widely spaced large lamellae).
Close to equilibrium. Fast diffusion. Coarse Pearlite. thicker layers.
high undercooling and faster cooling
Fast nucleation, slow growth = slow overall reaction.
Many nuclei that grow slowly (closely spaced fine lamellae)
very fine pearlite. Layers very close together
rapid cooling (water quench)
Supersaturated solid solution which forms martensite at lower temperature. Unstable austenite solid solution which forms martensite at lower temperature.
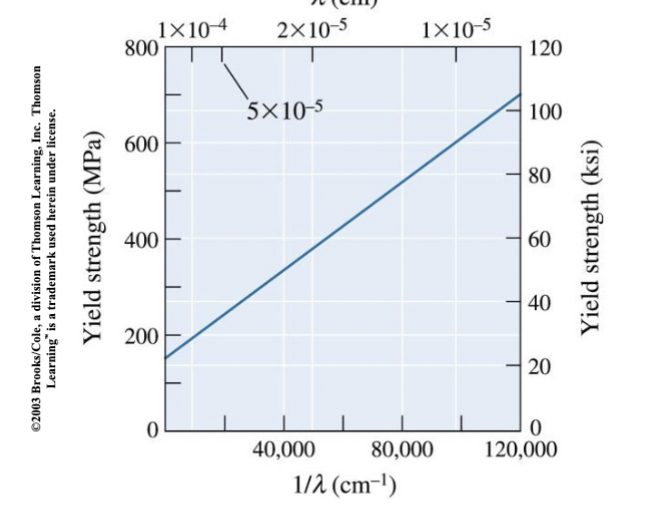
lamellar spacing
of pearlite, depends on how far carbon can diffuse in the time available during cooling.
Graph shows yield strength vs inverse of this in pearlite.
decreased ________ → higher yield strength
fraction pearlite
equal to the fraction of austenite (g) before the transformation when cooling at Fe-0.3wt%C alloy.
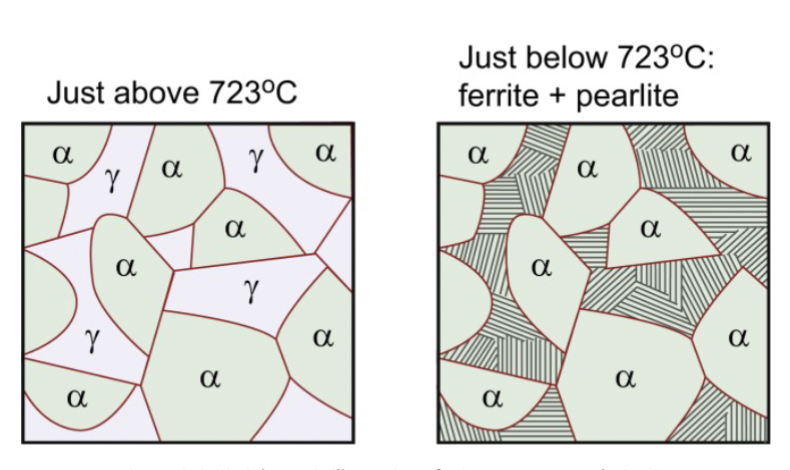
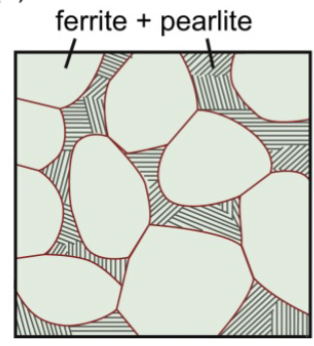
hypoeutectoid steel
from ferrite and austenite to ferrite and pearlite
microstructure in hypoeutectoid steel
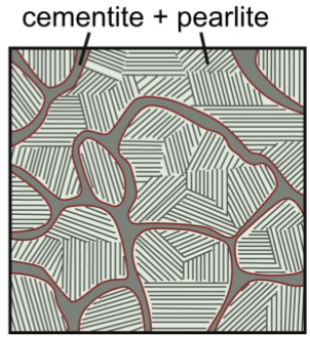
hypereutectoid steel
microstructure composition constists mainly of colonies of pearlite with some proeutectoid cementite; means at a higher composition of carbon than a regular eutectoid reaction
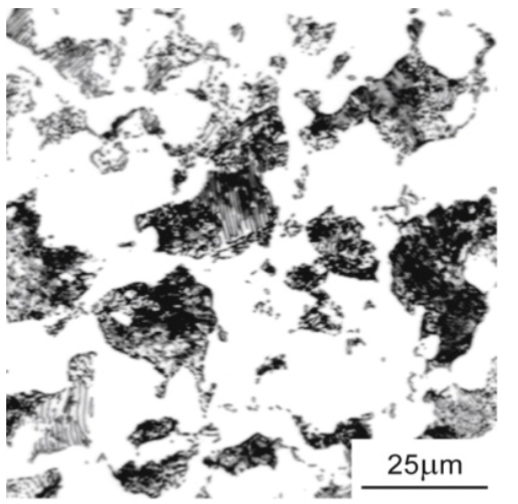
hypo
0.2wt%C - ferrite + pearlite
microstructure gives a higher ductility; almost all ferrite. <0.8%. Good ductility and strength
hyper
1.0wt% C: cementite and pearlite. >0.8%. microstructure contains more cementite, which is a brittle phase. Very strong but not as ductile. more Fe3C → network causes to be more brittle
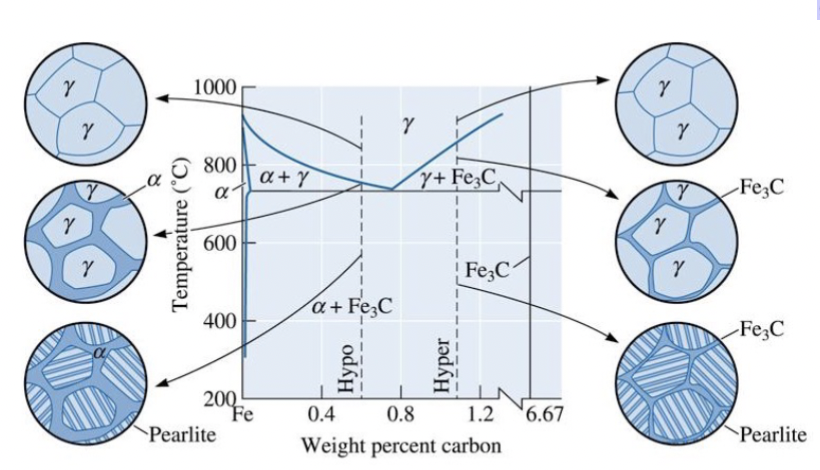
evolution of the microstructures
The ____________________ of hypoeutectoid and hypereutectoid steels during cooling in relationship to the Fe3C phase diagram.
Increasing pearlite amounts converge at the eutectoid point.
composition (wt%C)
Controlling Amount of Eutectoid Microconstituent: By changing this, we change the amount of pearlite
cooling rates
Controlling the Lamellar Spacing and Strength: Lamellar spacing and strength are controlled by this. Higher ________ lead to finer lamellae and greater strength
time-temperature-transformation (TTT)
diagram for eutectoid steel. Logarithmic scale. PS: start of pearlite formation. Pf: finish of transformation to pearlite (consume all austenite).
Below 550 ℃ → avoid forming pearlite.
γu : unstable → will transform, depending on temperature.
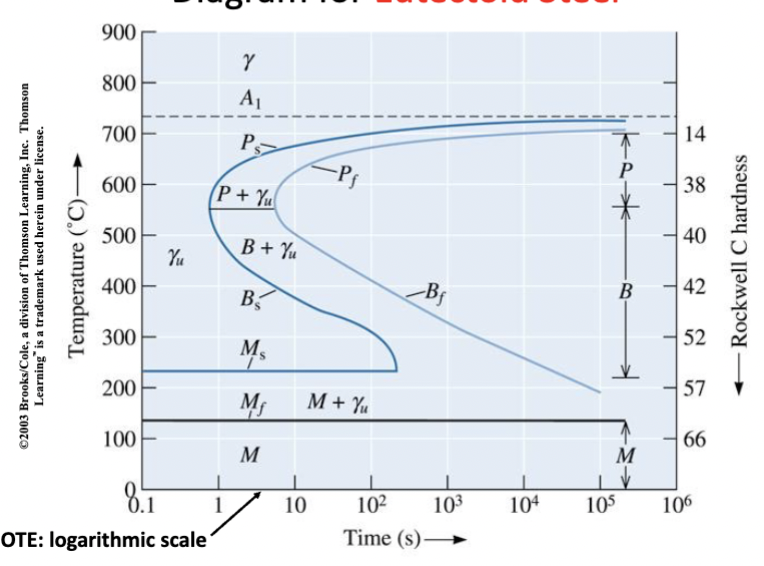
martensite
formation occurs without diffusion. Function of temperature only (not time). very hard and strong. Quite brittle. All carbon is in solution. Not at equilibrium so not on phase diagram.
Nucleation from austenite grain boundaries. Athermal transformation - no diffusion!