chapter 3 - the molecules of cells
1/74
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
75 Terms
carbon
element that is most used when making molecules
almost all molecules a cell makes are composed of carbon atoms bonded to one another and to atoms of other elements
carbon has the ability to form large and complex molecules that build structures and carry out functions
the carbon atom has 4 electrons in its valence shell that holds 8, meaning it has the ability to form FOUR covalent bonds
organic compounds
carbon-based molecules (that usually contain hydrogen atoms as well)
its shape usually determines its function
the atoms attached to the carbon skeleton of an organic compound affects its properties
carbon skeletons
carbon chains that form the backbone of most molecules
they differ in length and can be straight, branched, or arranged in rings (where each corner represents a carbon and it’s hydrogen)
can include double bonds which can differ in number and location
they can have the same chemical formula (C4H10 for butane and isobutane) but differ in arrangement
applies to double bonds as they would have a different 3-D shape due to its location
isomers
compounds with the same formula but different structural arrangements
add greatly to the diversity of organic molecules and its properties
*can result from the different spatial arrangements that can occur when four different properties are bonded to a carbon atom
important in pharmaceuticals because the two isomers may not be equally effective or could lead to harmful/dangerous effects
hydrocarbons
molecules only consisting of only carbon or hydrogen
provides much of the world’s energy
rare in living organisms but hydrocarbon chains are found in regions of molecules
ex. fat contains chains that provide fuel to the body
length in carbon skeletons
carbon skeletons can vary in length in which depends on the amount of carbons (more carbons, the more longer)
double bonds in carbon skeletons
carbon skeletons may have carbon that are double bonded together that vary in location but include the same amount of carbons, but less hydrogen because there are less valence capacity for those carbon atoms that are double bonded
branching in carbon skeletons
carbon skeletons may be branched or not, meaning that carbons may be bonded to the backbone that extended from it in another direction, like branches from the trunk of a tree
rings in carbon skeletons
carbon skeletons may be arranged in to rings which are abbreviated ring structures where each corner of the ring represents a carbon and it’s hydrogens unless stated otherwise
functional groups
groups that affect a molecule’s function by participating in chemical reaction
these groups are usually polar (the methyl group is not polar and not reactive, meaning its hydrophobic, but affects the shape and function of the molecule), allowing for them to be hydrophilic
soluble in water, making it necessary for life
hydroxyl group
[-OH]
consists of a hydrogen atom bonded to an oxygen atom
alcohols are organic compounds that contain hydroxyl groups (ex. ethanol)
carbonyl group
[>C=O]
consists of a carbon atom double bonded to an oxygen stom
can be located within or at the end of a carbon skeleton
simple sugars contain carbonyl groups
carboxyl group
[-COOH]
consists of a carbon atom double bonded to an oxygen atom and also bonded to a hydroxyl group
can act as an acid by contributing H+ to solutions (becoming ionized)
called carboxylic acids
amino group
[-NH2]
consists of a nitrogen atom bonded to two hydrogen atoms
can act as a based by absorbing H+ from a solution (and becoming ionized)
called amines (amino acids — amino group and carboxyl group)
phosphate group
[-OPO32-]
consists of a phosphorus atom bonded to four oxygen atoms (usually ionized)
called organic phosphates and are often involved in energy transfers (energy rich compound —> adenosine triphosphate)
methyl group
[-CH3]
consists of a carbon bonded to three hydrogen atoms
methylated compounds (a component of DNA) affects the expression of genes
four main classes of molecules in all living things
carbohydrates
lipids
protiens
nucleic acids
macromolecules
gigantic molecules
carbohydrates, proteins, and nucleic acids
ex. protein molecules can have over 1,000 atoms
polymers
chains formed by joining smaller molecules to make macromolecules (many parts)
a long molecule consisting of many identical or similar building blocks strung together
monomers
the building blocks of polymers
dehydration reaction
a reaction that removed a molecule of water as two molecules become bonded together (forming polymers with monomers)
each monomer contributes part of the water molecule that is released during the reaction
one monomer loses a hydroxyl group and the other loses a hydrogen atom to form H2O or water
new covalent bonds form to link the two monomers to create a polymer
*this process is the same regardless of the different monomers and the type of polymer the cell is producing
hydrolysis
the breaking of polymers with water molecules into monomers
the bonds between monomers are broken by the addition of water molecules
hydroxyl group attaches to one monomer
the hydrogen atom attaches to another
most organic molecules are in food and are often in the form of polymers (too large and is needed to be digested)
ex. lactose-intolerant people are unable to hydrolyze those bonds in sugar lactose because they lack the enzyme, lactase
enzymes
specialized macromolecules that speed up chemical reactions in cells
is needed for dehydration reaction and hydrolysis
the diversity of polymers
a cell makes thousands of different macromolecules from a small lost of ingredients (elements)
40-50 components and a few rare others
ex. proteins are built from 20 types of amino acids and DNA is made up from only 4 types of monomers called nucleotides
*the arrangement of monomers in polymers leads to diversity with the variation in the sequence in which monomers are strung together
variety in polymers leads to the uniqueness of each organism though monomers themselves are universal
SMALL MOLECULES COMMON TO ALL ORGANISMS ARE ORDERED INTO LARGE MOLECULES, WHOCH VARY SPECIES TO SPECIES AND INDIVIDUALS WITHIN A SPECIES
carbohydrates
the class of molecules that range from small sugar molecules to large polysaccharides
4 jobs
stores energy
provide structure
cellular identity
building block for other acids
almost all carbohydrates are hydrophilic owning to the many hydroxyl groups attached to sugar monomers
monosaccharides
simple sugars that act as the monomers of carbohydrates
they can be hooked together through dehydration reactions to form more complex sugars and polysaccharides
ex. monosaccharides in honey are glucose and frutose
has molecular formulas that are multiples of CH2O
glucose and fructose are common monosaccharides that both have a molecular formula of C6H12O6 and contains multiple hydroxyl and carbonyl groups (they are isomers with the same formula but different arrangement - the position of carbonyl groups in this case)
minor differences give isomers varying properties and in how they react with other molecules
has between 3-7 carbons
5-carbon sugars are called pentoses
6-carbon sugars are called hexoses
5-6 carbon sugars form rings in aqueous solutions versus maintaining their linear skeletons
glucose: carbon 1 bonds to the oxygen of oxygen 5, with carbon 6 extending above the ring
disaccharides
formed by cells with monosaccharides undergoing a dehydration reaction
sucrose is the most common
made up of a glucose monomer linked to a fructose monomer
maltose is another example
made up of two glucose monomers linked together
polysaccharides
macromolecules that are polymers of hundreds to thousands of monosaccharides linked together through dehydration reaction
they can function as storage molecules or as structural compounds
starch
a storage polysaccharide in plants that consists of long chains of glucose monomers
coils into a helical shape and may be unbranched or branched
served as carbohydrate “banks” from which plant cells can withdraw glucose for energy or building materials
*humans and animals have enzymes that can hydrolyze plant starch into glucose (plants include potatoes, rice, wheat, and corn)
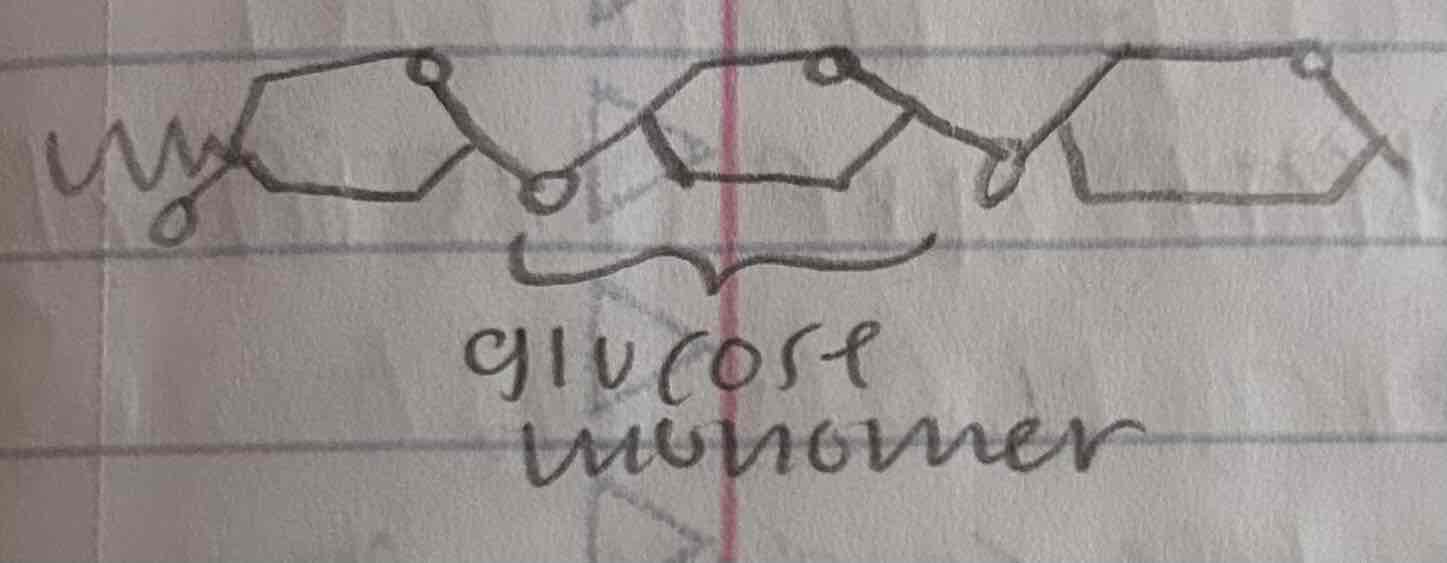
glycogen
a storage polysaccaride where animals store glucose
more branched than starch
glycogen is stored as granules in the liver and the muscle cells in which hydrolyze the glycogen to release glucose when needed
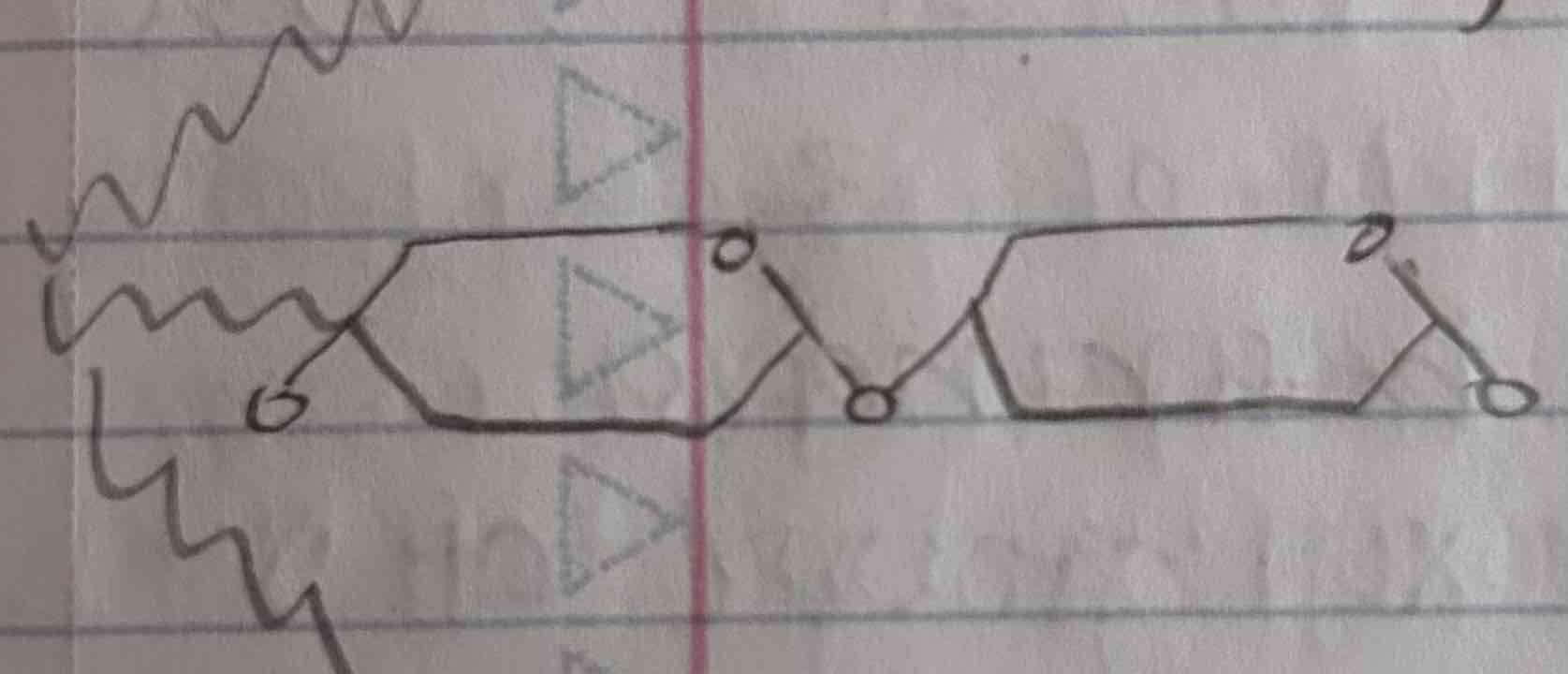
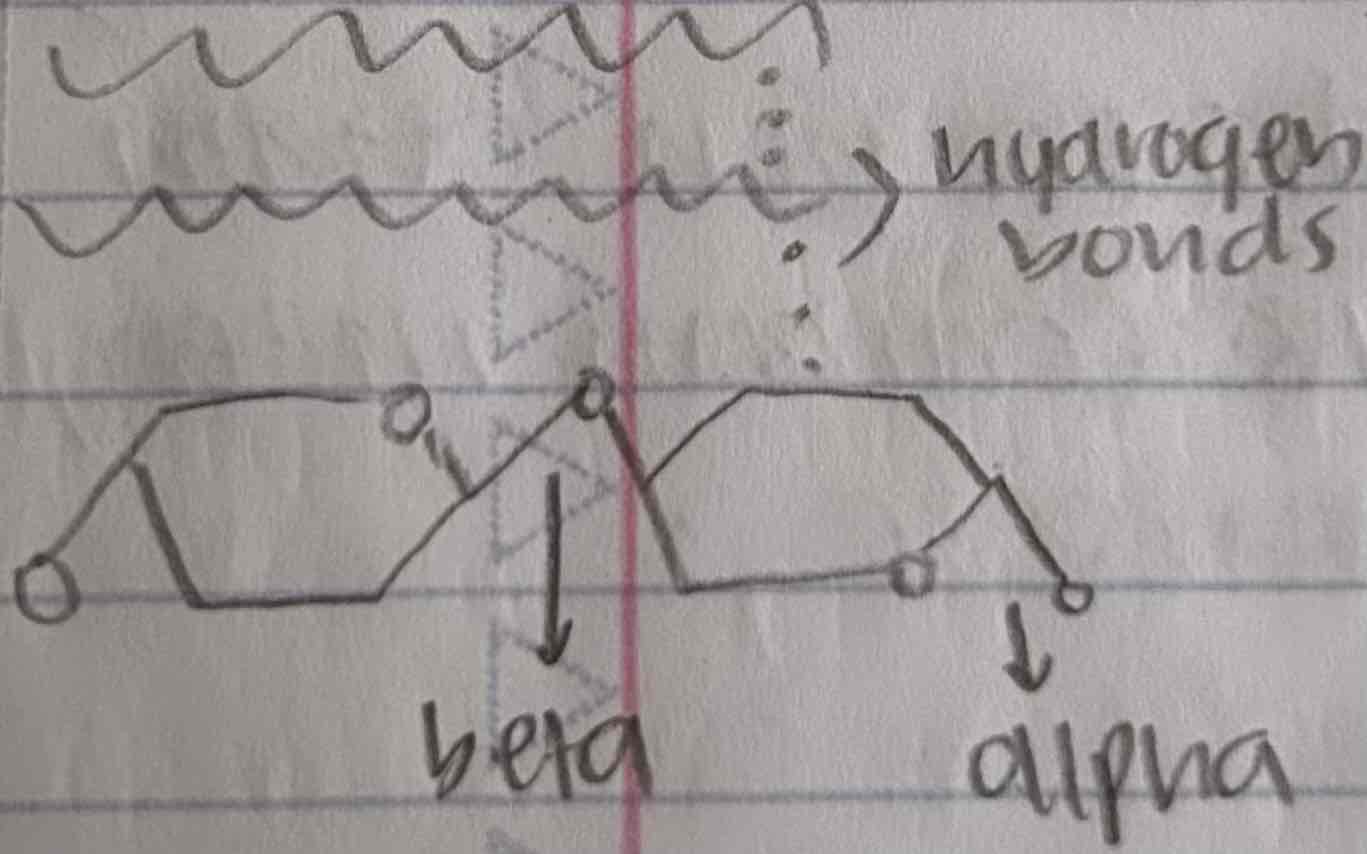
celluose
the most abundant organic compound which is a major component of the tough walls that enclose plant cells
polymer of glucose, but its monomers are linked together in a different oriantation
when arranged parallel to each other, they are joined by hydrogen bonds (forming cable-like microfibrils that combine with other molecules)
*animals cannot hydrolyze cellulose as it is insoluble but it does not act as a nutrient but contributes to digestive health as a fiber
cows and termites has microorganisms that can hydrolyze cellulose and fungi can hydrolyze cellulose as well
chitin
a structural polysaccharide used by insects and crustaceans to build their exoskeletons (a hard case enclosing an animal)
found in the cell walls of fungi
lipids
a diverse group of molecules that are classified together because they share one trait of not mixing well with water (hydrophobic —> water fearing: due to nonpolar C - H bonds in the hydrocarbon chains)
not huge macromolecules nor polymers built from similar monomers (different from carbohydrates, proteins, and nucleic acids)
three types of lipids
fats
phospholipids
steroids
fats
a large lipid made from a glycerol molecule and 3 fatty acid molecules through dehydration reaction
fats store energy as it is easiest for it to be stored in the form of fat but harder to burn of excess (a reasonable amount is normal and healthy)
stored longer term in adipose cells
cushions vital organs snd insulates the body
glycerol
consists of 3 carbons, each bearing hydroxyl group
major component of lipids
fatty acids
consists of a carboxyl group and a hydrocarbon chain (16-18 carbons in length)
major component of lipids
unsaturated fatty acids
a fatty acid whose hydrocarbon chain contains one or more double bonds
each carbon atom connected by a double bond has one fewer hydrogen atoms attached to it which causes kinks in the hydrocarbon chain
unsaturated fats has a lower melting point and is usually more of a liquid as its kinks in its fatty acids allow for less surface area for intermolecular forces to occur
fats of plants and fish are generally unsaturated as their kinks make for them to be a liquid at room temperature (called oils)
saturated fatty acids
a fatty acid that had no double bonds in its hydrocarbon chain meaning that it has the maximum number of hydrogen atoms attached to each carbon atom
its carbons are “saturated” with hydrogen
saturated fats have a higher melting point as its fatty acids have a larger surface area with no kinks allowing for stronger molecular forces to occur (london dispersion)
most animal fats are saturated as the hydrocarbon chains are packed closely together due to a lack of double bonds, making them solid at room temperature, like butter
trans fats
converted unsaturated fatty acids into saturated fatty acids by adding hydrogen by hydrogenation
partially hydrogenated oils
*commonly associated with health risks and is banned by the FDA
phospholipids
contains two fatty acids attached to glycerol instead of three where a phosphate group that is negatively charges is attached to glycerol’s third carbon instead
is a major component of cell membranes (cells couldn’t exist without it)
the ends of phospholipids have different relationships with water (they form a double-layered sheet in water)
hydrophobic tails are in the center which is excluded from the water
hydrophilic heads are on the other side of the membrane which is in contact with water
steroids
lipids in which the carbon skeleton contains four fused rings
cholestrol
a common part of animal cell membranes and is a steroid
also the precursor (substance that precedes and is transformed into another substance —> complex molecules) for making other steroid
steroids vary in the chemical groups attached to the rings
ex. sex hormones
anabolic steroids
synthetic variants of the male hormone, testosterone, that mimics its effects
anabolic —> anabolism: the building of substances in the body
proteins
a polymer of small building blocks called amino acids
the most structurally and functionally varied
nearly every function in the body depends on proteins
enzymatic proteins
selective acceleration of chemical reactions (enzymes are chemical catalysts)
ex. digestive enzymes
*most important role
defensive proteins
protection against disease
ex. anti-bodies inactive and destroy viruses and bacterias
storage proteins
storage of amino acids and supply amino acids to embryos
ex. proteins found in eggs and seeds
transport proteins
transportation of substances
hemoglobin transports oxygen from the lungs to the body and others transports molecules through cell membranes
hormonal proteins
coordination of an organism’s energy
insulin
receptor proteins
the response of a cell to chemical stimuli
ex. signaling pathways, receiving and transmitting, into cells
contractive proteins
contraction and movement
in muscle cells to contract
structural proteins
to support and form structure
fibers in tendons and ligaments (connective tissues)
shape of proteins
a protein’s shape determines its function
order of amino acids or 3-D shape
*proteins are usually just a long chain of amino acids
two models
ribbon model
space-filling model
globular
fibrous
denaturation
when a protein is altered when it unravels (losing its shape) thus its function is affected and the protein loses its job
can be due to excessive heath (ex. frying an egg — the yolk)
renaturation
when denatured proteins fold back into its functional shape
only occurs in the proper environment
prions
proteins that do not fold back correctly
an accumulation of misfolded proteins are associated with serious and degenerative brain diseases such as alzheimer’s and parkinson’s)
amino acids
monomers of proteins
has one amino group and a carboxyl group that are both covalently bonded with a central carbon atom
other partners bonded to the central carbon atom is a hydrogen atom and a “R”-group
R-groups
determine the amino acid’s function
radical as it varies from amino acids to amino acid
hydrophilic amino acids
when the amino acid is polar or charged
meaning the R-groups contain oxygen and nitrogen
often ionized forms as if they contain acids or basic groups, they are charged to the pH of the cell
hydrophobic amino acids
when the amino acid is nonpolar
meaning that the R-groups contain carbon and hydrogen (similar electronegativity)
peptide bonds
covalent linkage resulting of amino acids bonding together in a dehydration reaction that links the carboxyl group of one amino acid to the amino group of another
dipeptide
two amino acids bonded
polypeptide
more than two amino acids bonded together
primary structure
the precise sequence of amino acids in the polypeptide chain which are connected by peptide bonds
secondary structure
segments of the polypeptide chain that then coil or fold into local patterns
hydrogen bonds between atoms of the polypeptide backbone (oxygen to hydrogen)
two forms: alpha helix and beta pleated sheet
quaternary structure
more than one polypeptide bonds in a 3-D structure in which subunits are held together by interactions between R-groups
4 identical polypeptides (subunits) form a functional protein
gene
a discrete unit of inheritance
consists of DNA
nucleic acids
polymers
“nucleic” comes from the location of DNA in cells
nucleotides
monomers of nucleic acids
each of DNA’s nucleotides has 4 nitrogenous bases
adenine, thymine, cytosine, guanine
*genetic information is written in a four letter alphabet
each of RNA’s nucleotides has 4 nitrogenous bases
A, C, G, and uracil instead of T
polynucleotides
a nucleic acid polymer that is built from its monomers by dehydration reaction
the sugar of one nucleotide bonds to the phosphate group of the next monomer
results is a repeating sugar-phosphate backbone in the polymer (nitrogenous bases are not apart of the backbone)
RNA
ribonucleic acid
consists of a single polypeptide strand
assembles polypeptides according to instructions by DNA
base pairings can occur between the stretches on complementary nucleotides
takes on 3-D shapes for specific functions
3 types of RNA are involved in protein synthesis
DNA
deoxyribonucleic acid
consists of two polypeptide strands that wind around each other forming a double helix
the nitrogenous bases protrude from the backbone and pair in the center of the helix with hydrogen bonds (A with T, C with G)
the genetic material that humans and all other organisms inherit their parents consist of DNA
it resides in a cell as one or more long structures called chromosomes (which carries several hundred and more genes)
provides directions for its own replication (every time a cell divides, it makes an identical copy of its chromosomes)
the double helix “unzips” and new complementary strands assemble along the separated strands (thanks to base pairing)
*these instructions program all of a cell’s activity by directing the synthesis of proeins
base-pairing rules
the two strands are to be complementary (be a predictable counterpart of the other)
gene expression
the flow of genetic information in the building of a protein — also known as protein synthesis
*DNA is transcribed into RNA which is than translated to form the protein
gene directs the synthesis of an RNA molecule
DNA is transmitted into RNA (same pairing rules apply but A with U because it is RNA)
RNA molecule interacts with the protein building machinery of the cell
the information is translated from “nucleic acid language” to “protein language” which is the amino acid sequence of a polypeptide
*main idea: an organism’s genes determine the proteins —> structure and function of the body
lactose tolerance
a mutation as human stop producing the enzyme in early childhood and lactose intolerance is the norm
mutations are examples of convergent evoltuton
a similar adaptation evolving independently in different lineages