Chapter 8--Aromatic Compounds
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
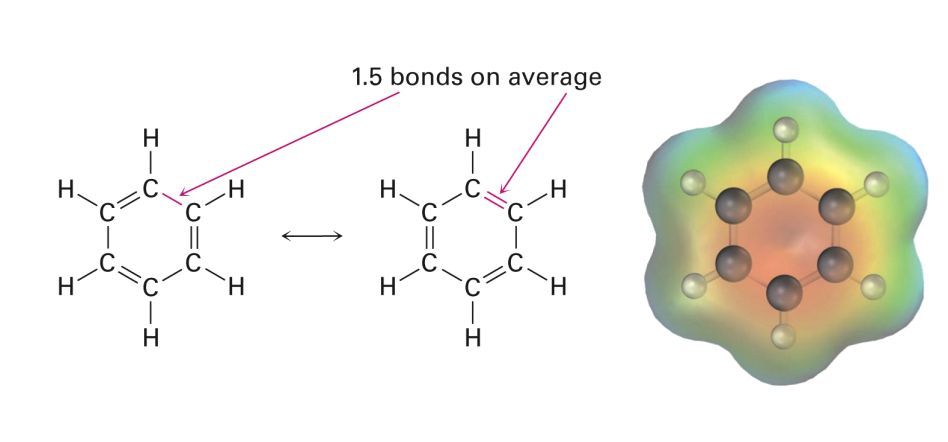
The Structure of Benzene
Naming Benzene Derivatives
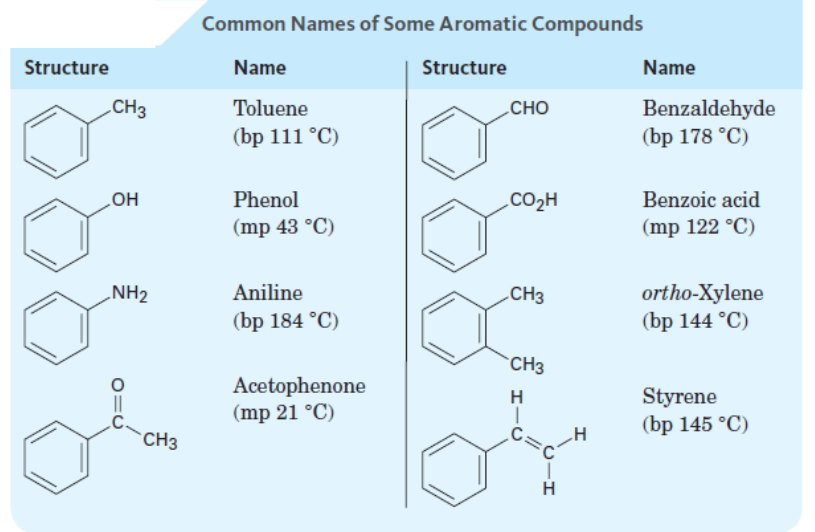
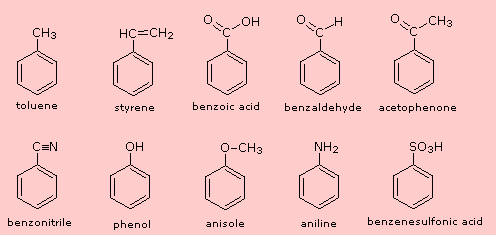
IUPAC discourages use of common names, except those of aromatic compounds
• Parent can be a common name (table 5.1) or named as "-benzene"
• Substituent in common name automatically gets numbered
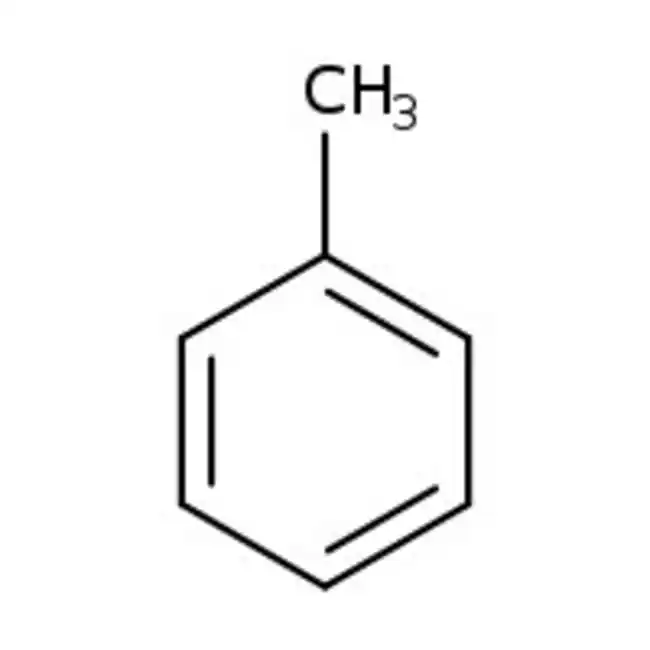
toulene
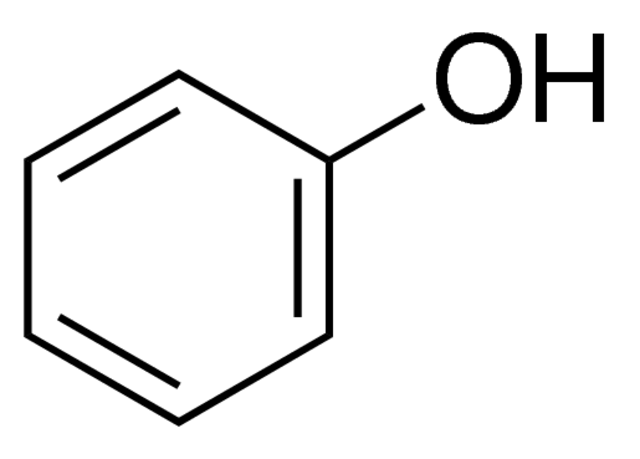
phenol
benzoic acid
Naming Benzene Derivatives
Benzene Substitution Patterns
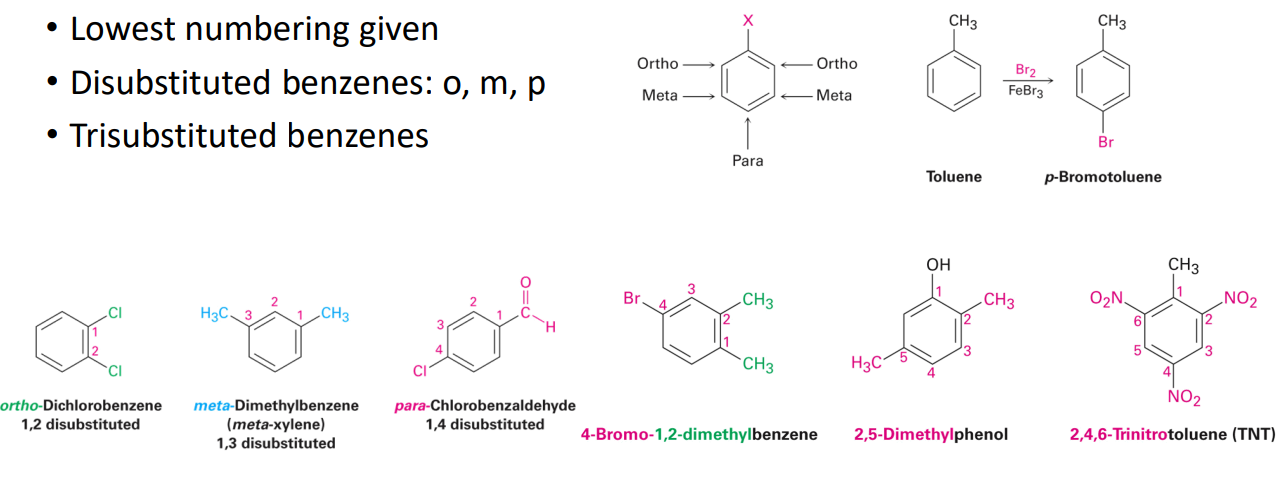
Disubstituted benzenes are named using one of the prefixes ortho- (o), meta- (m), or para- (p).
(Ortho: think position 1 and 2 on hexene ring)
(Meta: think positions 1 and 3 on hexene ring)
(Para: think position 1 and 4 on hexene ring)
Naming Benzene Derivatives
Lowest numbering given
Disubstituted benzenes: o, m, p
Trisubstituted benzenes
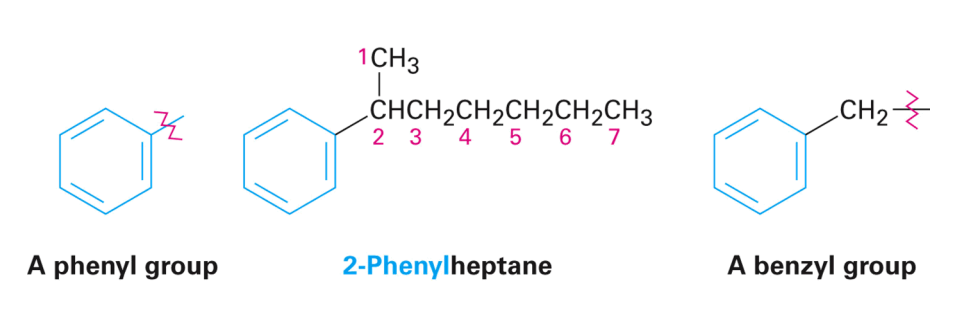
Phenyl vs. Benzyl
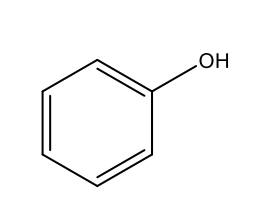
What is the IUPAC name of the following structure?
phenol
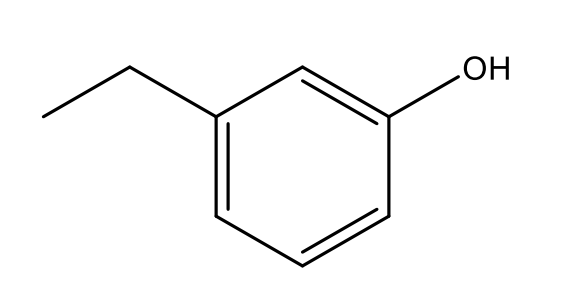
What is the IUPAC name of the following structure?
m-ethylphenol
(position 1 and 3 → meta. Ethyl on 1, but no need for numbering. And phenol is the hexene ring with an OH group)
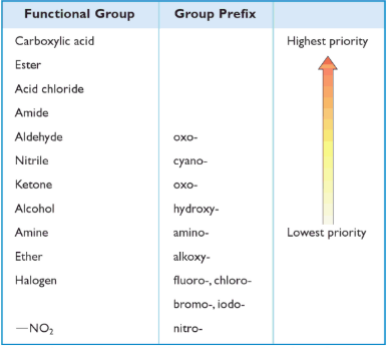
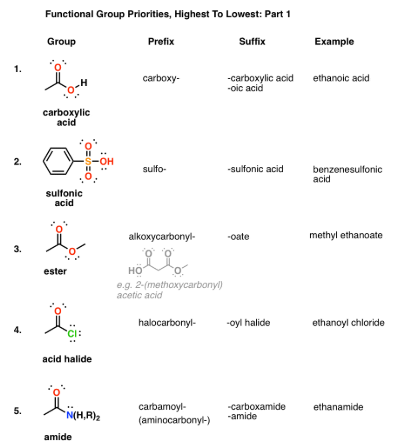
Functional groups, group prefixes, and priority levels
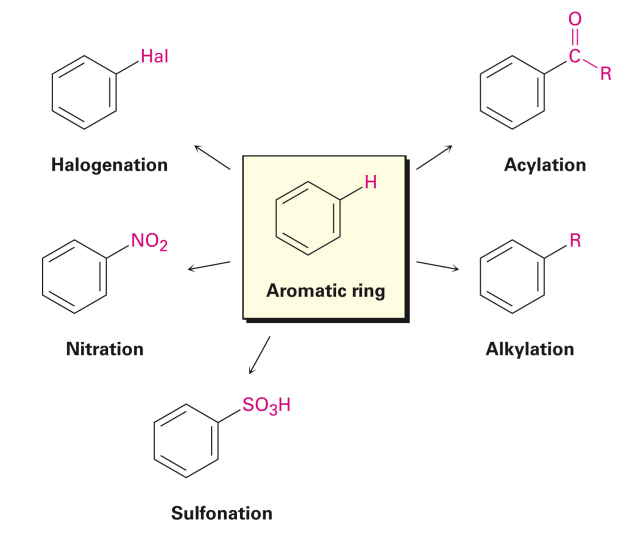
Electrophilic Aromatic Substitution Reactions
EAS
an electrophile (E+) reacts with benzene (nucleophile)
Bromination
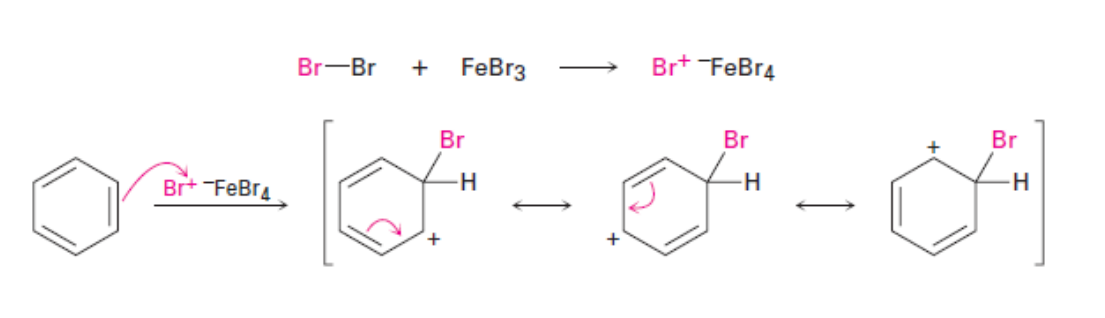
Bromination process
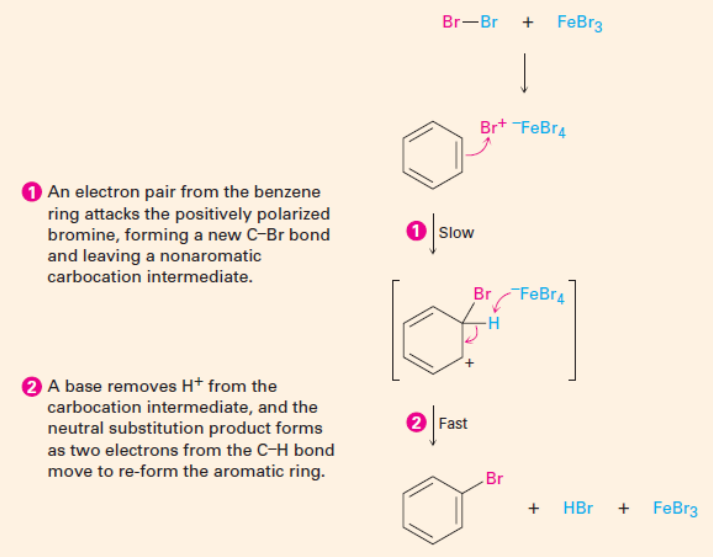
EAS Nitration
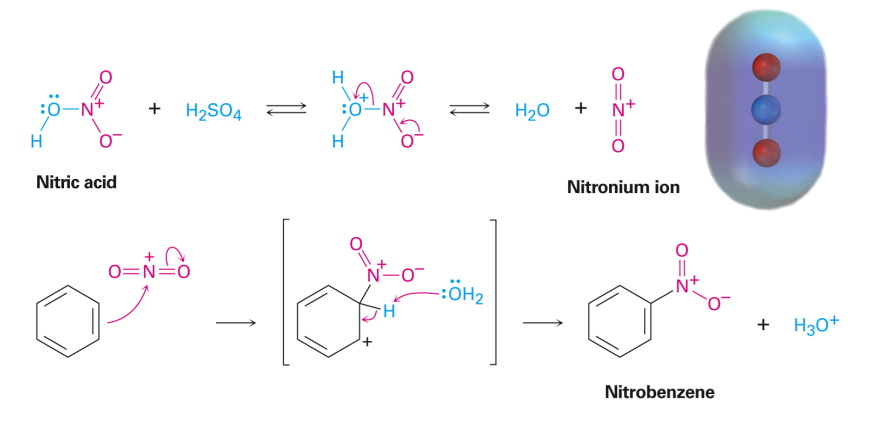
Sulfonation
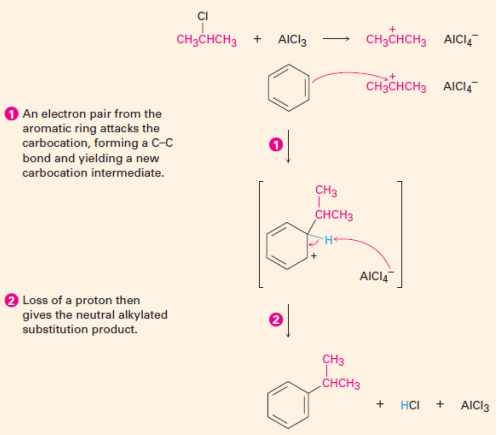
Friedel-Crafts Alkylation
Alkylation introduces an alkyl group to the ring
Electrophile is a carbocation
Limitations: 2 degree and 3 degree alkyl halides need to be used if there are 3 or more carbons; electron withdrawing group (EWG) on benzene ring → Friedel Crafts won’t work
Friedel Crafts Alkylation
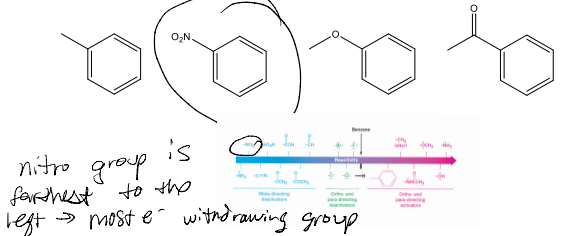
Substituent Effects in EAS
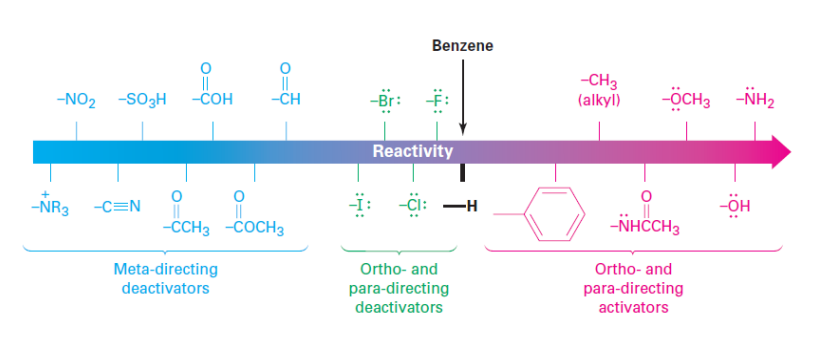
• Substituents affect the reactivity of the ring
• Substituents affect the regioselectivity of the reaction (o, m, p)
• Adding substituents may make a material much more or much less reactive than unsubstituted benzene in EAS.
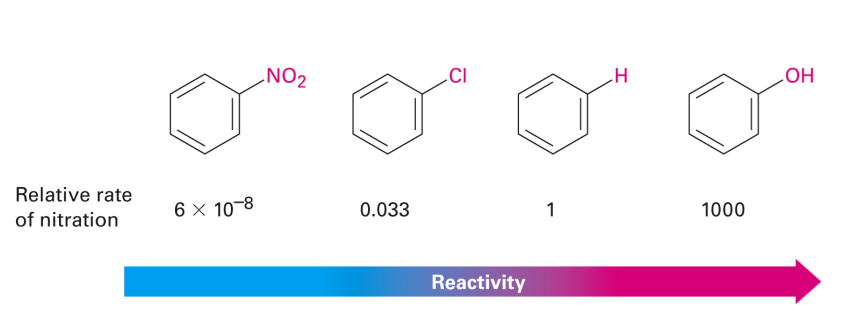
Substituent Effects: Reactivity graph
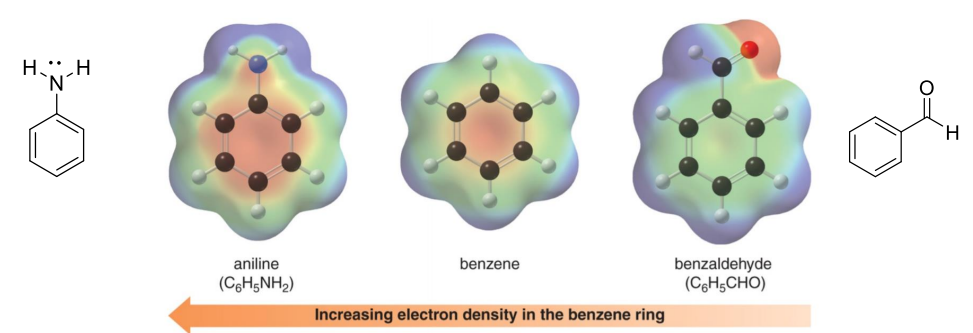
• Aniline has increased electron density in benzene ring.
• Benzaldehyde has decreased electron density in benzene ring.
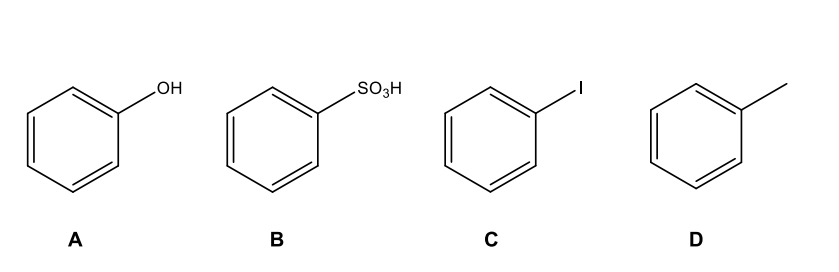
Which of the following will NOT work in a Friedel-Crafts acylation reaction?
B
Phenol stability
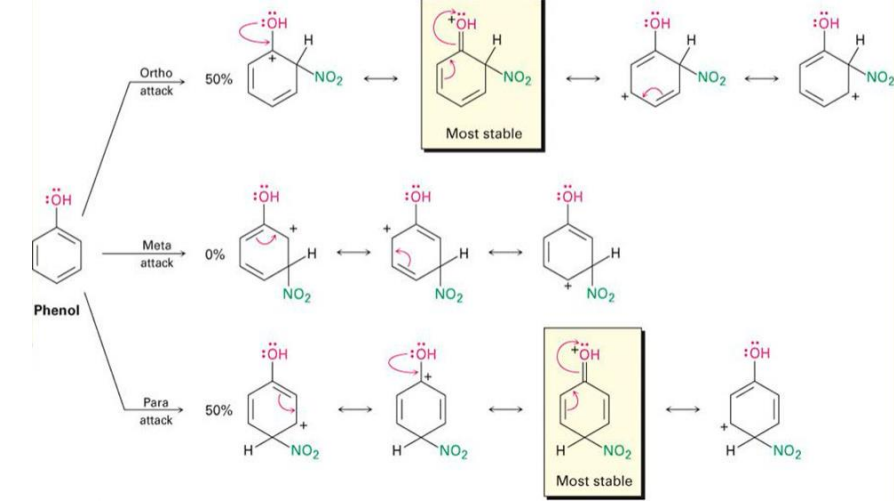
Toulene stability

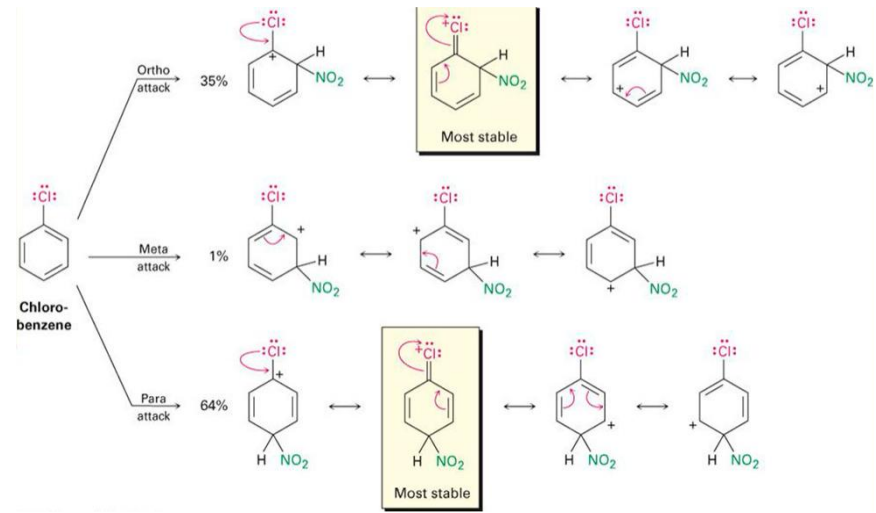
Chloro-benzene stability
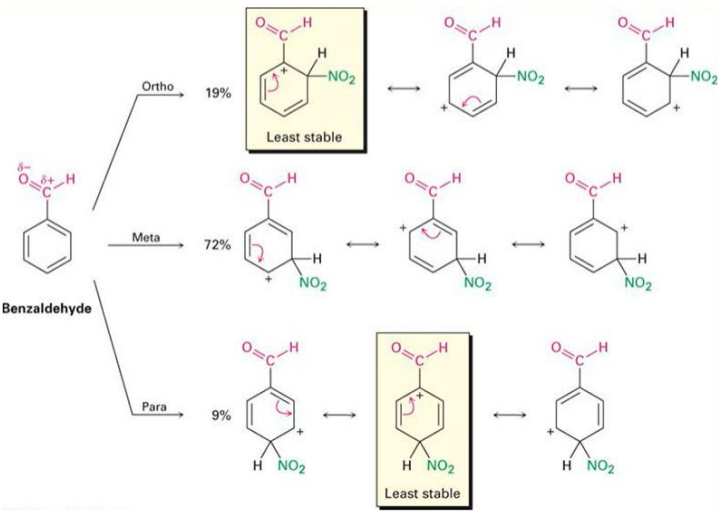
Benzaldehyde
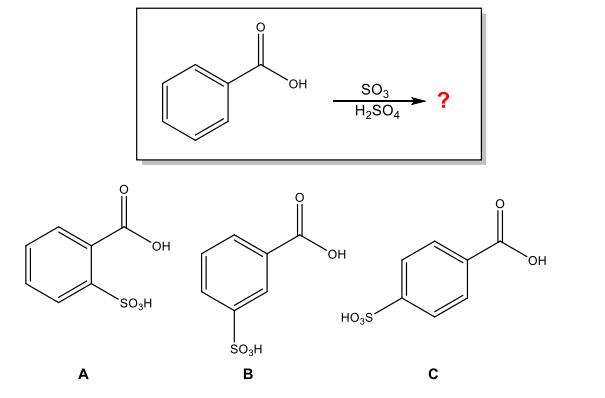
What is the major organic product of the following reaction?
B
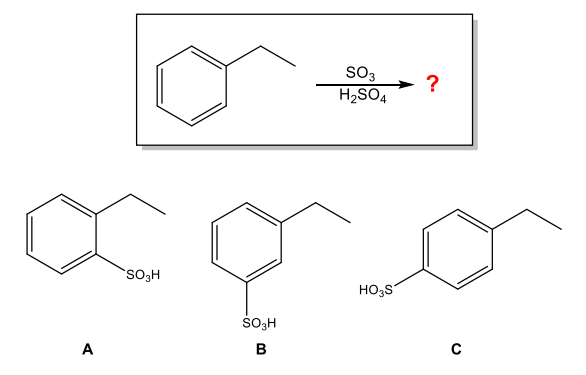
What is the major organic product of the following reaction?
A and C (ortho and para)
Heterocycle
contain elements other than carbon in a ring (N, S, O, P)
Molecule structures
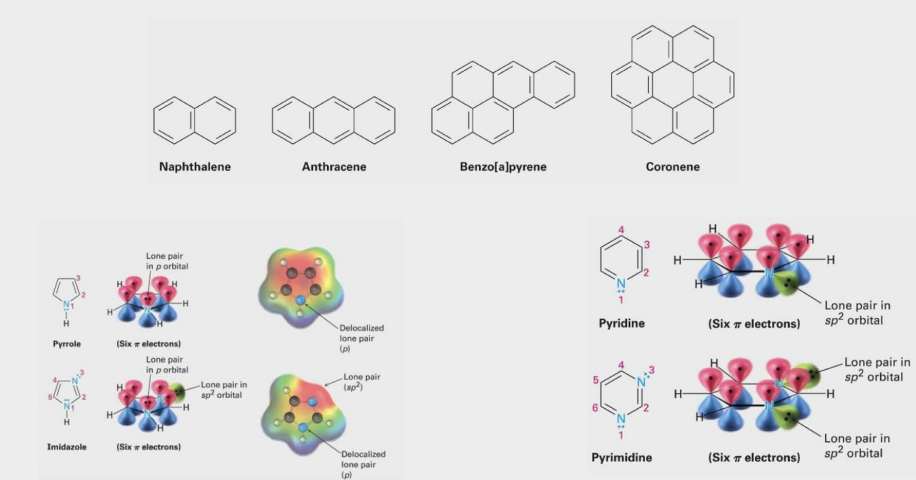
Combined names
Singular names
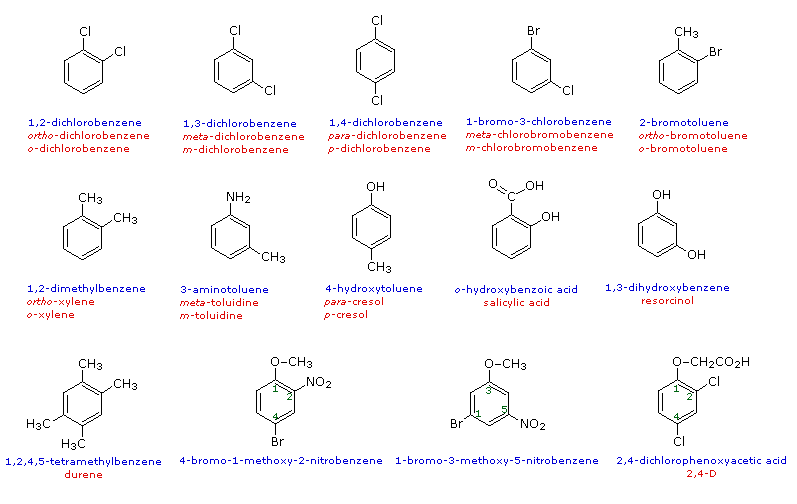
When do we use ortho, meta, and para?
When more than one substituent is present on a benzene ring, the relative locations of the substituents must be designated by numbering the ring carbons or by some other notation. In the case of disubstituted benzenes, the prefixes ortho, meta & para are commonly used to indicate a 1,2- or 1,3- or 1,4- relationship respectively. In the following examples, the first row of compounds show this usage in red. Some disubstituted toluenes have singular names (e.g. xylene, cresol & toluidine) and their isomers are normally designated by the ortho, meta or para prefix. A few disubstituted benzenes have singular names given to specific isomers (e.g. salicylic acid & resorcinol). Finally, if there are three or more substituent groups, the ring is numbered in such a way as to assign the substituents the lowest possible numbers, as illustrated by the last row of examples. The substituents are listed alphabetically in the final name. If the substitution is symmetrical (third example from the left) the numbering corresponds to the alphabetical order.
Highest Priority Groups: Carboxylic Acids, Sulfonic Acids, Esters, Acid Halides, Amides
Next In Line: Nitrile, Aldehyde, Ketone, Alcohol, Thiol, Amine
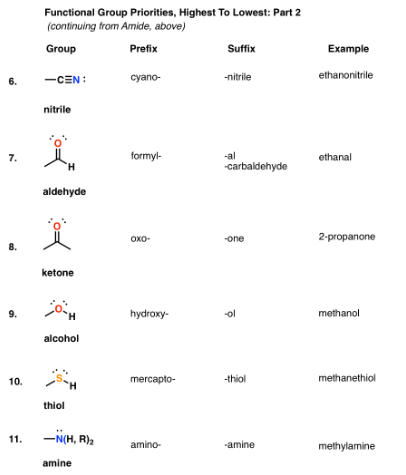
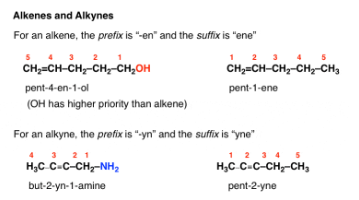
Alkenes And Alkynes naming
For the purposes of the name, “-ene” comes before “-yne” alphabetically. So when an alkene and an alkyne are present in the same molecule, the ending will always be “yne”.
For the purposes of numbering, if there is a tie between an alkene and an alkyne for determining the lowest locant, the alkene takes priority.
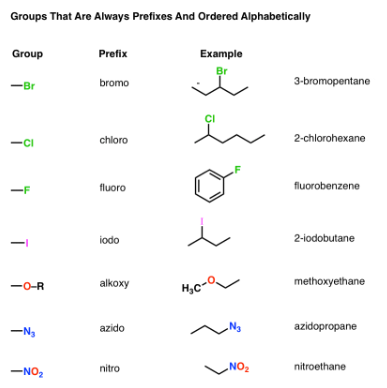
Functional Groups That Are Always Prefixes: Halides, Alkoxides, Azides, Nitro
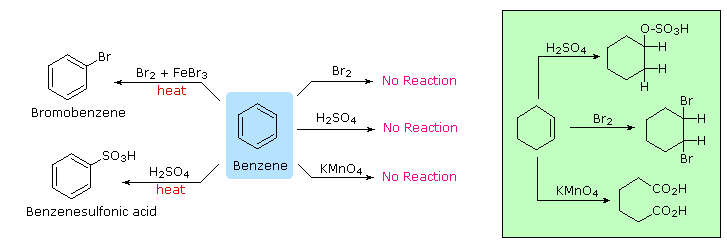
Substitution Reactions of Benzene and Other Aromatic Compounds
The remarkable stability of the unsaturated hydrocarbon benzene has been discussed in an earlier section. The chemical reactivity of benzene contrasts with that of the alkenes in that substitution reactions occur in preference to addition reactions, as illustrated in the following diagram (some comparable reactions of cyclohexene are shown in the green box).
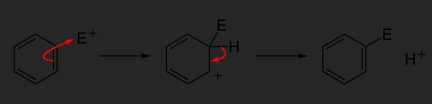
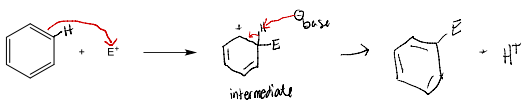
EAS reactions all follow the same general two-step mechanism.
Step 1: An electrophile attracts the pi electrons of one of the double bonds of the aromatic benzene ring which results in the formation of a carbocation. This step is slow.
Step 2: The carbocation intermediate loses a proton. These electrons are used to reform a pi bond and restore aromaticity. As opposed to the first step, this step is fast because aromaticity is regained. It is important to note that the carbocation loses a proton where the electrophile attacked the benzene ring.
The Five Most Common EAS Reactions:
1. Halogenation: Functional groups: halides (Br, Cl). Requires a specific catalyst: FeCl3 for chlorination and FeBr3 for bromination.
2. Sulfonation: This is the only reversible EAS reaction. The reagent is concentrated sulfuric acid.
3. Nitration: Nitric acid is the reagent and sulfuric acid is the catalyst. Both are required to prepare the electrophile.
3. Nitration: Nitric acid is the reagent and sulfuric acid is the catalyst. Both are required to prepare the electrophile.
5. Friedel-Crafts acylation: the initial acyl chloride can have any R group instead of methyl
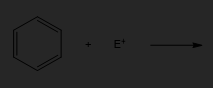
Draw the arrow pushing mechanism showing each step and the products for the electrophilic aromatic substitution of benzene with a generic electrophile, E+.
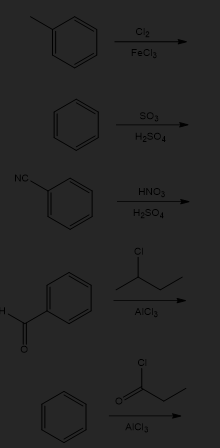
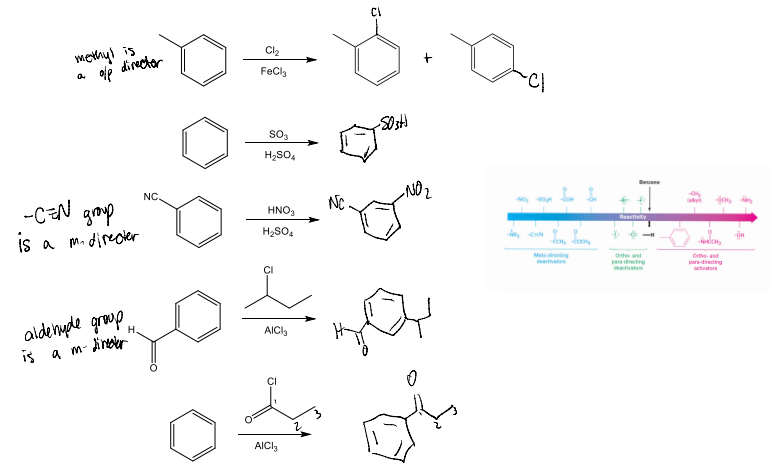
Predict the major product(s) of the following reactions:
Which of the following compounds would react the slowest towards chlorination?