MI580 Lecture 4 - Observational Studies
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
Observational Studies
Patients are ‘observed’ in normal clinical practice (‘real world setting’), self-assigned exposures.
Subject to a number of biases.
Real World Data
Data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources
Real World Evidence
The clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of RWD
Ecologic Studies
Observational studies of population group characteristics.
Cannot demonstrate causality. Mostly focused on hypothesis generating, not testing.
Ecological Fallacy
Assigning the characteristics of a group to individuals within the group who may not have that characteristic
Case-Series/Case Study
Prevalence survey, typically involves small numbers of subjects. No comparison or control group.
Often used to characterize rare diseases.
Cross-Sectional Studies
An observational study in which exposure and disease (or disease outcome) are determined at the same point in time in a given population.
Can show possible exposure/outcomes associations, but cannot determine causality due to lack of temporal data.
AKA Prevalence study
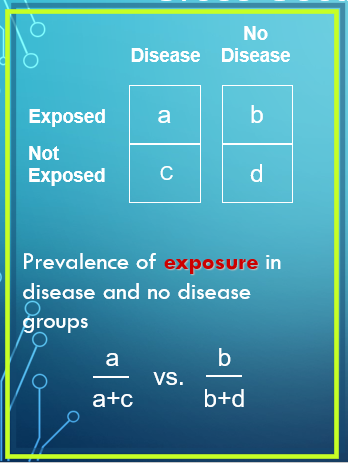
The Four Possible Categories in a Cross-Sectional Study
Exposed, have disease
Exposed, no disease
Not exposed, have disease
Not exposed, no disease
Prevalence of Exposure in Cross-Sectional Study Calculation
Prevalence of Disease in Cross-Sectional Study

Cohort studies
Exposure-based subject selection, longitudinal studies. Groups followed for outcomes.
Can be retrospective or prospective.
Steps to Design a Cohort Study (3)
Define population of interest
Identify exposure status of individuals
Follow over time for outcomes
Advantages of Prospective Cohort Study (3)
Can ensure good measurements of exposure
Can ensure relatively accurate measure of disease onset dates
Can add new measures over time as new information on risks and exposures becomes available
Limitations of Prospective Cohort Studies (3)
Time-consuming
Expensive
Biases from nonresponse and loss to follow-up
Retrospective Cohort Study
Investigator uses existing historical data collected previously to identify the population and the exposure status (exposed vs non-exposed groups).
Determine current patient status with no follow up.
Retrospective Cohort Study Advantages (2)
Typically quick to establish cohort within an existing database (e.g. EMR, registers, or insurance claims database)
Relatively inexpensive
Retrospective Cohort Study Limitations (2)
Must rely on “pre-recorded” data or memories
Data may not contain accurate diagnoses; chart abstraction or other means of validation may be necessary for outcome (and possibly exposure)
When to Choose Retrospective Cohort Study
Exposure and outcome have already occurred, medical history for both exposure and outcome is considered accurate
When to Choose Prospective Cohort Study
Neither exposure nor outcome have occurred or only exposure has occurred, medical history information is inadequate to capture outcome
Advantages of Cohort Studies (4)
Ability to explore many outcomes
Permit calculation of direct risk
Can study uncommon exposures
Can study temporal relationship between exposure and health outcome
Disadvantages of Cohort Studies (4)
Expensive
Time-consuming (prospective)
Not ideal for rare diseases
Lost to follow-up
When a Cohort Study is Warranted (4)
When the (alleged) exposure is known
When exposure is rare and incidence of disease among exposed is high
When the time between exposure and disease is relatively short
When adequate funding is available
Case Control studies
Cases and controls are selected based on outcome, then sampled for previous exposures.
If the exposure is in fact related to the disease, then it is anticipated that the prevalence of history of exposure in the cases will be greater than the prevalence of history of exposure in the controls
How to Identify Cases for Case-Control Studies (5)
Self-report
Hospital records
Patient charts
Laboratory records
Surveillance data
How to Select Case-Control Controls
Conceptually, controls should come from the same population at risk of disease from which cases develop.
But practically, controls are often selected to be similar to cases on key factors but without the disease.
Matching in Case-Control Studies
Matching controls to cases on potential confounders or key characteristics (e.g., age, sex, ethnicity)
Recall Bias in Case-Control Study
Information on some past exposures depends on memory of events from both cases and controls, which can be flawed.
Recall bias occurs when the recall is better among cases than controls because of the presence of disease
Case-Control Study Advantages (3)
Can measure multiple exposures for the disease
More efficient than a cohort study for rare outcomes
Costs relatively less and typically can be conducted in a shorter time
Case-Control Study Disadvantages (4)
Must have a fairly common exposure
Can’t always confirm timing of exposure
Higher chance of certain biases
Provides indirect estimate of risk