RNA modifications
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
How many RNA modification are there?
~100, more than DNA, chemically diverse
Where do modifications occur?
On tRNAs and rRNAs, which are abundant in cells so easy to detect. Also detected in mRNAs using next generational sequencing.
Most heavily modified RNA molecules
tRNAs
How often do tRNA modifications occur?
Every few nucleotides, more chemically diverse than DNA modifications
Most heavily modified site
position 34 of tRNAs - wobble position
What is the wobble position?
3rd position of anticodon site in tRNA
Recognise more than 1 codon in mRNA.
The G recognises both C and U by nucleotide modifications
Allow non-standard Watson-Crick base pairing
example in human mtRNA
mitochondrial disease due to mutation in enzyme that catalyses the modification. Mutation - not catalysed.
Normal wobble modification in methionine (human mitochondrial tRNA)
cytosine → 5-formyl cytosine
Important bc they encode methionine so initiators for translation
CAU recognises AUA and AUG
Enzyme for cytosine → 5-methylcytosine
(In methionine mt-tRNA C34)
NSUN3
Enzyme for 5-methylcytosine → 5-formylcytosine
ABH1
What happens if NSUN3 not present/mutated?
5-methylcytosine and 5-formylcytosine no longer form
What happens when 5-formylcyotosine not present?
Initiation of translation is inhibited
Leads to mitochondrial dysfunction
What is the epitranscriptome?
chemical modifications on RNA
Most prevalent modification type in vertebrate epitranscriptome
methyl-6-Adenosine then 5mC and inosine
How are they detected?
NGS
Process of transcriptome sequencing
extract + purify mRNA → convert to DNA using reverse transcriptase → PCR amplify → sequence
Problem with process of transcriptome sequencing
RNA modifications lost when converted to DNA
Method used to map 6-methyladenosine
m6A-Seq
How does m6A-Seq work?
fragmentation - manipulated so they are a certain size (100nt) → purify m6A fragments with immunoprecipitation with anti m6a antibody → sequencing → gives 100nt windows of there m6a occurs.
Where do m6a marks cluster?
start of 3’UTRs, after the stop codon
Which proteins bind to m6a and what do they do?
YTHDF1 → elevates translation
YTHDF2 → decay of mRNA
What does this mean?
When m6a is present, get a short burst of translations
Why is this important?
e.g. synapses need short bursts of signalling
Why is mapping of 5mc challenging?
Not as abundant as m6a
Why is bisulphite sequencing inaccurate in RNA?
You get incomplete conversions → false positives
Methods to map 5mc
bisulphite sequencing
similar to m6a-seq
Aza-IP
methylation iCLIP
3 and 4 are cross linking methods
How do catalytic cross linking methods work?
induce a cross-link between the enzyme catalysing the modification
incubate mRNA with 5-Aza-C → overexpresses methyltransferase → enzyme methylated → becomes cross-linked → sequencing detects cross-link
How does miCLIP work?
modify the enzyme → get a cross link when it methylates the cytosine → customise sequencing to map where methylation occurs.
Cross-link allows you to map to nucleotide resolution
Where does 5mc occur?
In the 3’UTR, probably has regulatory roles
How do inosine bases form?
via enzymatic modification of adenosine bases
Why is inosine special?
results in an edit in the coding sequence that can change the amino acids present in the translated protein
Which enzymes convert A to I
ADAR1 or ADAR2
What happens when AUA → AUI
I recognised as G, changes the amino acid.
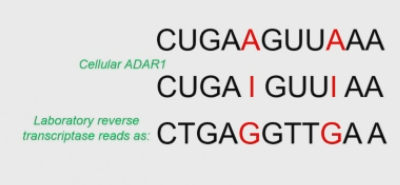
How is inosine detected?
Reverse transcriptases also read inosines as guanosines → there will be differences compared to the exprected sequence.
A→G substitution indicates an A→I conversion. rare in vertebrantes.
Why is inosine important in AMPA receptors?
inosine edit causes glutamine→arginine
Arginine sits in the channel to close it.
Regulates opening + closing of channel.
What happens without the edit in AMPA?
channel permanently open → hyperexcitability → epilepsy