Week 1 and 2 Review
1/2569
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
2570 Terms
Homeostasis
Process of maintaining the “constancy” of our internal environment
What % of body weight in an adult does total body water account for?
~60%
What are the 3 compartments that total body water is divided into?
Intracellular
Interstitial
Plasma
What fraction of total body water does intracellular fluid make up?
2/3
Interstitial Fluid
Immediate environment of individual cells
Cells draw their nutrients from and release their products into the interstitial fluid
Cannot be a large reservoir for nutrients or a large sink for metabolic products because its volume is less than half that of the cells that it serves
Well-being of individual cells depends heavily on the homeostatic mechanisms that regulate the composition of the interstitial fluid
Accomplished by continuously exposing the interstitial fluid to "fresh" circulating plasma fluid
As blood passes through capillaries, solutes exchange between plasma and interstitial fluid by diffusion
Result is that interstitial fluid tends to take on the composition of incoming blood
What % of blood volume do suspended blood cells occupy?
~40%
What are the 3 conditions provided by the cardiovascular system that are essential for regulating the composition of the interstitial fluid?
There must be adequate blood flow through the tissue capillaries
The chemical composition of the incoming (or arterial) blood must be controlled to be that which is optimal in the interstitial fluid
Diffusion distances between plasma and tissue cells must be short
No cell in the body is located farther than ~10 um from a capillary
Diffusion is a poor mechanism for moving substances from the capillaries of an organ to the capillaries of another organ than may be 1 m or more distant
Substances are transported between organs by convection - substances move along with blood flow because there are either dissolved or contained within blood
What condition must be present for fluid to flow through a tube?
Fluid flows through the tube only when the pressure at the inlet and outlet ends (Pi and Po) are unequal, when there is a pressure difference (ΔP )
Vascular Resistance
Measure of how difficult it is to make fluid flow through the tube (how much of a pressure difference it takes to cause a certain flow
Flow Equation

What are the 2 ways in which blood flow through any organ can be changed?
By changing the pressure difference across its vascular bed
By changing its vascular resistance
More often changes in flow due to changes in vascular resistance
Resistance to Flow Through a Cylindrical Tube
Resistance to flow through a cylindrical tube depends on radius and length of the tube and the viscosity of the fluid flowing through it
Small changes in internal radius have a huge influence on resistance to flow
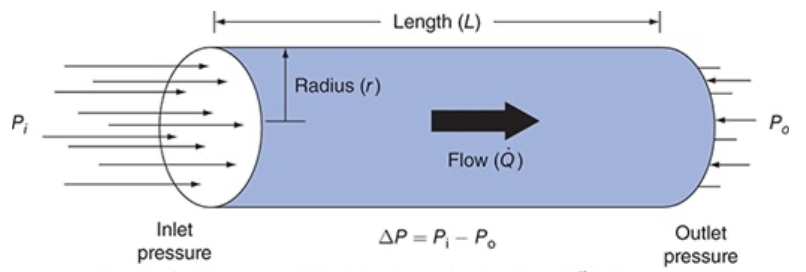
Resistance to Flow Through a Cylindrical Tube Equation
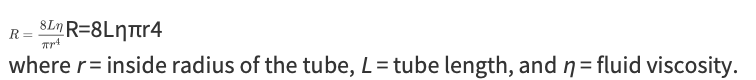
Poiseuille Equation
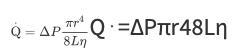
Regulation of Blood Flow Through Organs
Organ blood flows are regulated primarily through changes in the radii of vessels within organs
Vessel length and blood viscosity are not variable that can be easily manipulated for the purpose of moment-to-moment control of blood flow
Blood flows through vessels within an organ because a pressure difference exists between the blood in the arteries supplying the organ and the veins draining it
Normally average pressure in systemic arteries is approximately 100 mm Hg, and the average pressure in systemic veins is approximately 0 mm Hg
Convective Transport
Process of being swept along with the flow of the blood in which substances are contained
Rate at which the substance is transported by this process depends solely on the concentration of the substance in the blood and the blood flow rate
Transport Rate Equation
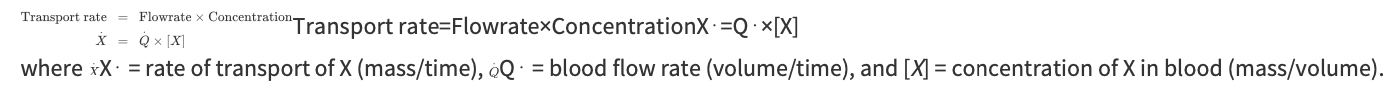
What are the two methods available for altering the rate at which a substance is carried to an organ?
A change in the blood flow rate through the organ
A change in the arterial blood concentration of the substance
What do you need to consider to calculate the rate at which a substance is being removed from (or added to) the blood as it passes through an organ?
To calculate the rate at which a substance is being removed from (or added to) the blood as it passes through an organ you have to simultaneously consider the rate at which the substance is entering the organ in the arterial blood and the rate at which the substance is leaving the organ in the venous blood
Transcapillary Efflux Rate Equation
Used to deduce a tissue's steady-state rate of consumption (or production) of any substance
When a substance enters a tissue from the blood it can either increase the concentration of itself within the tissue or be metabolized within the tissue
In the steady state, the rate of the substance's loss from blood within a tissue must equal its rate of metabolism within that tissue

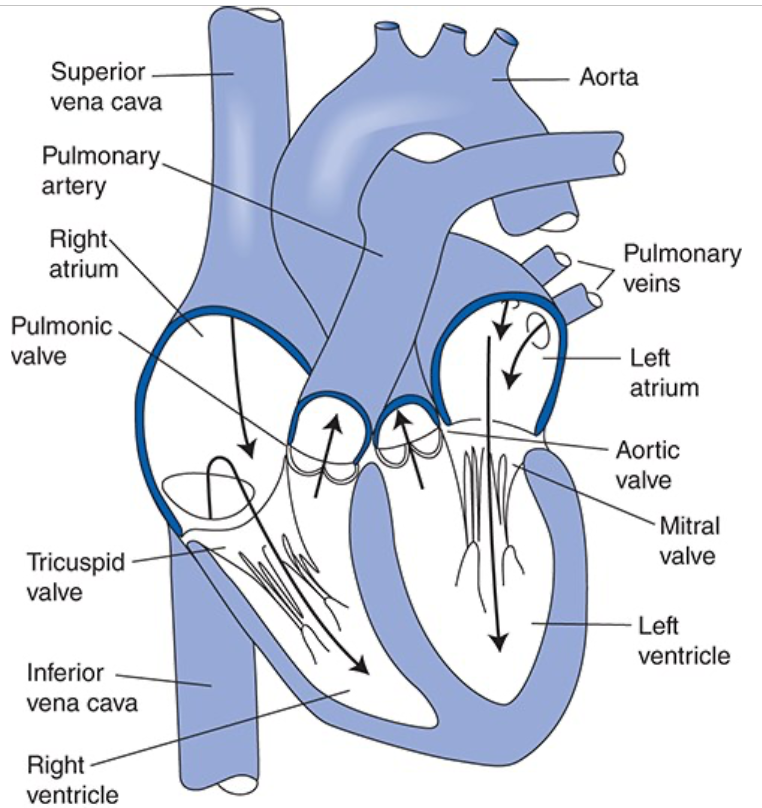
Chambers and Valves of the Heart and Pathway of Blood Flow Through Them
Venous blood returns from the systemic organs to the right atrium via the superior and inferior venae cavae
Passes through the tricuspid valve into the right ventricle and then is pumped through the pulmonic valve into the pulmonary circulation via the pulmonary arteries
Blood is reoxygenated in the capillaries of the lung
Oxygenated pulmonary venous blood flows in pulmonary veins to the left atrium and passes through the mitral valve into the left ventricle
Pumped through the aortic valve into the aorta to be distributed to the systemic organs
Pressure Changes Across the Heart Valves and in the Chambers that Lead to Blood Flow
Valves are structurally designed to allow flow in only one direction and passively open and close in response to the direction of the pressure differences across them
Ventricular pumping action occurs because the volume of the intraventricular chamber is cyclically changed by rhythmic and synchronized contraction and relaxation of the individual cardiac muscle cells that lie in a circumferential orientation within the ventricular wall
As soon as ventricular pressure exceeds the pressure in the pulmonary artery (right pump) or aorta (left pump) blood is forced out of the chamber through the outlet valve
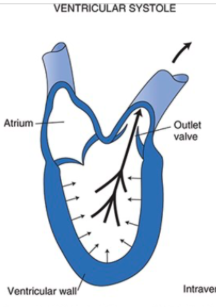
Systole
Phase of the cardiac cycle during which the ventricular muscles contract
Contraction of the ventricular muscle increases tension in the chordae tendinae which prevents the AV valve from opening despite the large pressure difference between the ventricle and atrium
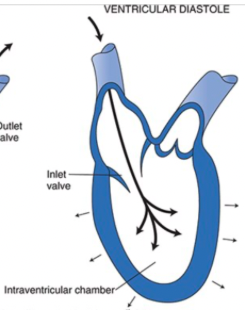
Diastole
When the ventricular muscle cells relax, the pressure in the ventricle falls below that in the atrium, the AV valve opens, and the ventricle refills with blood
The outlet valve is closed during diastole because arterial pressure is greater than intraventricular pressure
Cardiac Output Equation
CO determined by stroke volume and heart rate
Stroke volume - volume of blood ejected per beat
Heart rate - heart beats per minute
CO = SV x HR
Stroke Volume Equation
Stroke volume must equal the blood volume inside the ventricle at the end of diastole (end diastolic volume, EDV), minus ventricular volume at the end of systole (end systolic volume, ESV)
SV = EDV - ESV
How are action potentials conducted from one cell to the next?
Via gap junctions
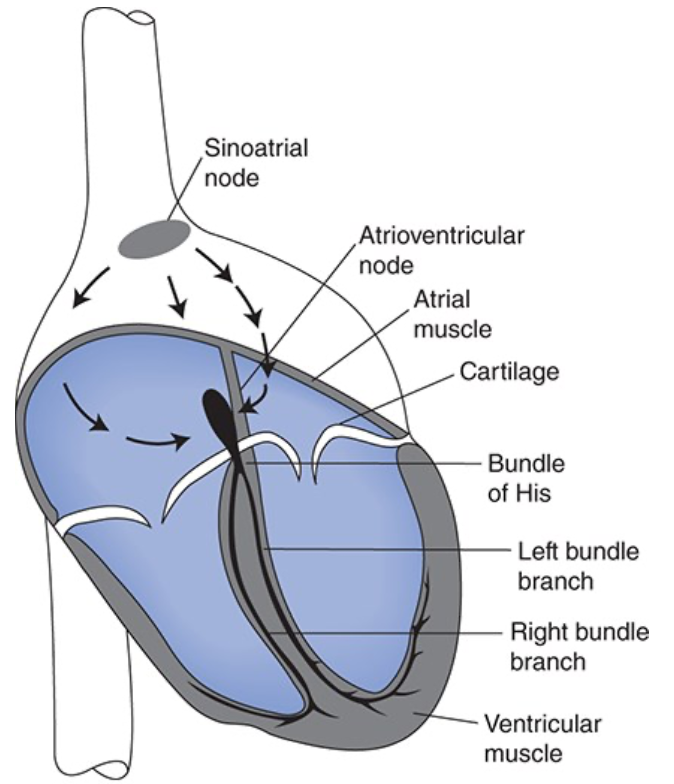
Components of the Cardiac Excitation and Conduction System
Components of excitation and conduction system
Sinoatrial node (SA node)
Contains cells that function as the heart's pacemaker and initiate the action potential
Atrioventricular node (AV node)
Contains slowly conducting cells that normally function to create a slight delay between atrial contraction and ventricular contraction
Bundle of His
Right and left bundle branches
Made up of Purkinje fibers
Specialized for rapid conduction and ensure that all ventricular cells contract at the same instant
What normally controls the heart rate?
Electrical activity of SA nodal cells
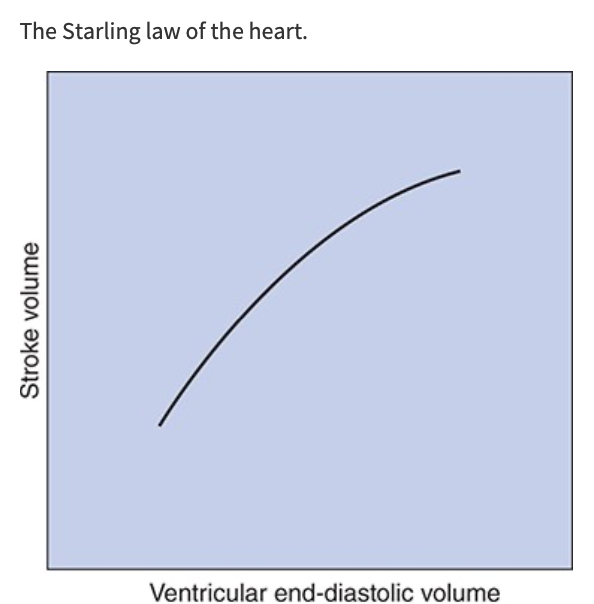
Starling Law of the Heart
With other factors being equal, if cardiac filling increases during diastole, the volume ejected during systole also increases, SV increases nearly in proportion to increases in EDV
Stroke volume, and therefore cardiac output, is strongly influenced by cardiac filling during diastole
Action of Sympathethetic Nerves on the Heart
Heart is innervated by adrenergic sympathetic fibers
When active, sympathetic nerves release norepinephrine on cardiac cells
Norepinephrine interacts with B1-adrenergic receptors on cardiac muscle cells
Increases HR
Increases the action potential conduction velocity
Increases the force of contraction and rates of contraction and relaxation
Overall, sympathetic activation acts to increase cardiac pumping
Action of the Parasympathetic Nerves on the Heart
Cholinergic parasympathetic fibers travel to the heart via the vagus nerve and innervate the SA node, the AV node, and the atrial muscle
When active, parasympathetic nerves release acetylcholine on cardiac muscle cells
Acetylcholine interacts with muscarinic receptors on cardiac muscle cells
Decreases the HR (SA node)
Decreases the action potential velocity (AV node)
May also decrease the force of contraction of atrial (not ventricular) muscle cells
Overall, parasympathetic activation acts to decrease cardiac pumping
Five Factors Essential to Proper Ventricular Pumping Action
The contractions of individual cardiac muscle cells must occur at regular intervals and be synchronized (not arrhythmic)
The valves must open fully (not stenotic)
The valves must not leak (not insufficient of regurgitant)
The muscle contractions must be forceful (not failing)
The ventricles must fill adequately during diastole
Arteries
Thick-walled
Contain some smooth muscle and a large component of elastin and collagen fibers
Can expand under increased pressure to accept and temporarily store some of the blood ejected by the heart during diastole and then, by passive recoil, supply this blood to the organs downstream during diastole
Arteries branch and although individual vessels get progressively smaller, the total cross-sectional area available for blood flow within the arterial system increases to several fold greater than the aorta
Often referred to as conduit vessels because they have relatively low and unchanging resistance to flow
Arterioles
Thicker walls with more smooth muscle and less elastic material
Due to muscle, diameters can be actively changed to regulate the blood flow through peripheral organs
Despite small size, arterioles are so numerous that in parallel, their collective cross-sectional area is much later than that at any level in arteries
Often referred to as resistance vessels because of high and changeable resistance, with regulates peripheral blood flow through individual organs
Capillaries
Smallest vessels in vasculature
Walls consists of a single layer of endothelial cells that separates the blood from the interstitial fluid by only 1 um
Contain no smooth muscle so lack the ability to change their diameters actively
So numerous that the total collective cross-sectional area of all the capillaries in systemic organs is more than 1000 times that of the root of the aorta
Viewed as the exchange vessels of the cardiovascular system
Veins
Thin walls in proportion to diameters
Contain smooth muscle and diameters can actively change
Very distensible so diameters change passively in response to small changes in transmural distending pressure
Have one-way valves that prevent reverse flow
Peripheral venules and veins normally contain more than 50% of the total blood volume
Thought of as capacitance vessels
Changes in venous volume greatly influence cardiac filling and therefore cardiac pumping so play an important role in controlling cardiac output
Effect of Sympathetic Nerves on Blood Flow through Vascular Beds
Blood flow through vascular beds is influenced by changes in activity of sympathetic nerves innervating arterioles
Nerves release norepinephrine that interacts with a-adrenergic receptors on the smooth muscle cells to cause contraction and arteriolar constriction
Reduction in arteriolar diameter increases vascular resistance and decreases blood flow
Most important means of reflex control of vascular resistance and organ blood flow
What affects arteriolar smooth muscle?
Arteriolar smooth muscle is responsive to changes in local chemical conditions and increased tissue metabolic rate leads to arteriolar dilation and increased tissue blood flow
Effect of Sympathetic Nerves on Venules and Veins
Venules and veins are innervated by sympathetic nerves and smooth muscle constricts when these nerves are activated
Increased sympathetic nerve activity is accompanied by decreased venous volume
Venous constriction decreases cardiac filling and therefore cardiac output
Hematocrit
Fraction of blood volume occupied by cells
Hematocrit = cell volume/total blood volume
What are the 3 formed elements in the blood and what are their functions?
Red cells - carry oxygen from the lungs to other tissues by binding oxygen to hemoglobin
White cells - immune function
Platelets - clotting
Plasma
Liquid component of blood
Inorganic electrolytes are the most concentrated solutes in plasma
Sodium and chloride are the most abundant and are responsible for plasma's osmolarity of ~300 mOsm/L
Contains many different proteins: albumins, globulins, fibrinogen
Do not readily cross capillary walls
Plasma concentrations are much higher than concnetration in interstitial fluid
Albumin most abundant
Serves as the vehicle for transporting nutrients and waste products
Contains many small organic molecules such as glucose, amino acids, urea, creatinine, and uric acid
Serum
Fluid obtained from a blood sample after its allowed to clot
Identical to plasma except it contains none of the clotting proteins
Pulmonary Circulation
Right heart pump and lungs
Systemic Circulation
Left heart pump supplying blood to systemic organs
Are the pulmonary and systemic circulations arranged in parallel or series?
Series, so right and left hearts must pump an identical volume of blood per minute (cardiac output)
Are organs arranged in parallel or series?
Most systemic organs are arranged in parallel within the cardiovascular system
Nearly all systemic organs receive blood of identical composition
Flow through any one of the systemic organs can be controlled independently of the flow through the other organs
Blood Conditioning Organs
Kidneys, skin, large abdominal organs
Can withstand a temporary severe reduction in blood flow because the amount of cardiac output they receive greatly exceeds the amount necessary to supply the nutrient needs of the tissue
Organs in Which Blood Flow Solely Supplies the Metabolic Needs of the Tissue
Brain, heart muscle, skeletal muscle
Do not tolerate blood flow interruptions well
Unconsciousness can occur within a few seconds after the stoppages of cerebral flow and permanent brain damage can occur in 4 minutes without flow
Heart muscle consumes approximately 75% of the oxygen supplied to it and the heart's pumping ability begins to deteriorate within beats of a coronary flow interruption
How do cardiac muscle cell action potentials differ from skeletal muscle cells?
They can be self-generating
They are conducted directly from cell to cell
They have long duration
How do ions move in and out of cells to make transmembrane potentials?
Ions are very insoluble in lipids so they cannot pass into or out of a cell through the lipid bilayer of the membrane but rather cross via protein structures embedded in the lipid cell wall
Ion channels
Responsible for resting membrane potential and for rapid changes in membrane potential that constitute the cardiac cell action potential
Ion exchangers
Ion pumps
How do concentration differences generate an electrical potential across a cell membrane?
K+ more concentrated inside the cell so K+ diffuses out of the cell
Negative charges, such as protein anions, cannot leave the cell because membrane isn't permeable to them
K+ efflux makes cytoplasm at the inside surface of the cell membrane more negative and the interstitial fluid just outside the cell membrane more positive
K+ is attracted to negative charge so electrical potential created across the membrane that tends to attract it back into the cell
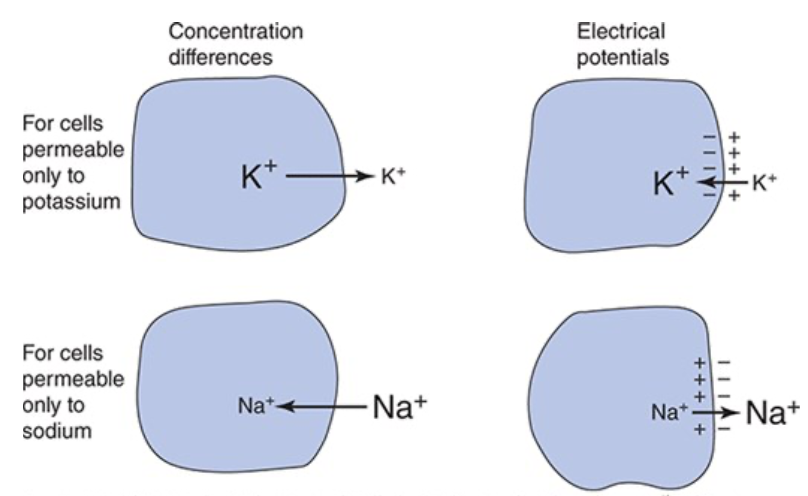
Equilibrium Potential
The electrical forces tending to pull an ion into the cell exactly balance the concentration forces tending to pull the ion out so there is no net movement of the ion
Equilibrium Potential for Potassium
-90 mV
Equilibrium Potential for Sodium
+70 mV
How does membrane potential reflect a membrane’s relative permeability to various ions?
Cell membranes are never permeable to just one ion so the membrane potential will lie somewhere between the equilibrium potential of the ions its permeable to, membrane potential will be closer to the ion it is more permeable to
Under resting conditions, most heart muscle cells have membrane potentials that are quite close to the potassium equilibrium potential
Action Potential
Result of large, rapid, and transient changes in the ionic permeability of the cell membrane that are triggered by an initial small, localized depolarization and then propagated over the entire cell membrane
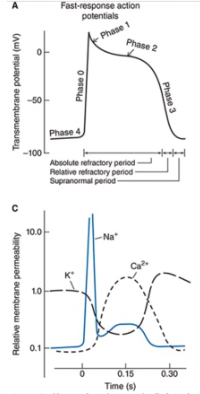
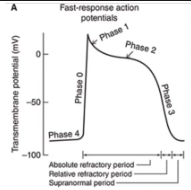
Fast Response Action Potentials
From ordinary cardiac muscle cells
Characterized by:
A rapid depolarization (phase 0) with a substantial overshoot (positive inside voltage)
A rapid reversal of the overshoot potential (phase 1)
A long plateau (phase 2)
A repolarization (phase 3) to a stable, high (large negative) resting membrane potential (phase 4)
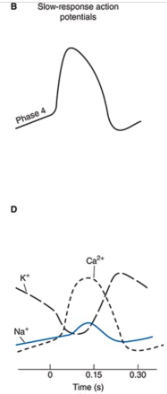
Slow Response Action Potentials
From cardiac pacemaker type cells
Characterized by a slower initial depolarization phase, a lower amplitude overshoot, a shorter and less stable plateau phase, and a repolarization to an unstable, slowly depolarizing "resting" potential
Unstable resting potential in pacemaker cells with slow-response action potentials is referred to as phase 4 depolarization, diastolic depolarization, or pacemaker potential
Cells usually found in the SA and AV nodes
When are the refractory periods of the cardiac cell electrical cycle?
Cells are in an absolute refractory state during most of the action potential - they cannot be stimulated to fire another action potential
Near the end of the action potential, membrane is relatively refractory - can be re-excited only by a larger than normal stimulus
Immediately after the action potential, the membrane is transiently hyperexcitable - "vulnerable" or "supranormal" period
Threshold Potential
Membrane potential that when depolarized to, results in major rapid alterations in permeability of the membrane to specific ions
What are 3 mechanisms that contribute to the slow depolarization of the membrane observed during the diastolic interval in pacemaker cells?
Progressive decrease in the membrane's permeability to K+ during the resting phase
Permeability to Na+ increases slowly
Slight increase in the permeability of the membrane to calcium ions late in diastole, resulting in an inward movement of the positive charged ions and contributes to diastolic depolarization
These mechanisms result in a specific current that occurs during diastole called the i-funny current
Phase 0
Rapid rising phase of fast-response action potential is a result of a sudden increase in Na+ permeability
Produces fast inward current of Na+
Causes membrane potential to move rapidly toward the sodium equilibrium potential
Short lived
Phase 1
Very brief increase in potassium permeability occurs that allows a transient outward-going potassium current (iTo) and results in a small non-sustained repolarization after the peak of the action potential
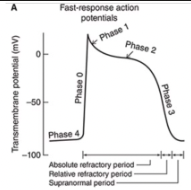
Phase 2
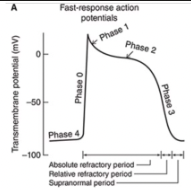
Prolonged depolarization plateau state is accomplished by interactions of two separate processes
Sustained reduction in K+ permeability
A slowly developed and sustained increase in the membrane's permeability to Ca2+
Under certain conditions, the electrogenic action of a Na+-Ca2+ exchanger may contribute to maintenance of the plateau phase of the cardiac action potential
Initial Depolarization in Slow-Response Action Potentials
Initial fast inward current is small or absent in cells that have slow-response action potentials
Initial depolarization phase is primarily a result of an inward movement of Ca2+ potentials
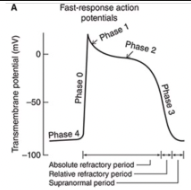
Phase 3
In both types of cells, the membrane is repolarized to its original resting potential as the K+ permeability increases to its high resting value and the Ca2+ and Na+ permeabilities return to their low resting values
What are the conformational states that ion channels exist in?
Open
Closed
Inactivated
Voltage Gated Channels
Probability of being open varies with membrane potential
Ligand-Gated Channels
Activated by certain neurotransmitters or other specific signal molecules
K+ Channel (Inward Rectifier), Kir Current
iK1
K+ Channel (Inward Rectifier), Kir Gating Mechanism
Voltage
K+ Channel (Inward Rectifier), Kir Functional Role
Maintains high K+ permeability during phase 4
Its decay contributes to diastolic depolarization
Its suppression during phases 0-2 contributes to plateau
Na+ Channel (Fast) Nav 1.5 Current
iNa
Na+ Channel (Fast) Nav 1.5 Gating Mechanism
Voltage
Na+ Channel (Fast) Nav 1.5 Functional Roles
Accounts for phase 0 of action potential
Inactivation may contribute to phase 1 of action potential
K+ Channel (transient outward), Kto Current
iTo
K+ Channel (transient outward), Kto Gating Mechanism
Voltage
K+ Channel (transient outward), Kto Functional Role
Contributes to phase 1 of action potential
Ca2+ Channel (Slow Inward, L Channels) Cav 1.2 Current
iCa
Ca2+ Channel (Slow Inward, L Channels) Cav 1.2 Gating Mechanism
Both
Ca2+ Channel (Slow Inward, L Channels) Cav 1.2 Functional Roles
Contributes to phase 2 of action potential
Inactivation may contribute to phase 3 of action potential
Is enhanced by sympathetic stimulation and B-adrenergic agents
K+ Channels (Delayed Rectifier), Ks, Kr, Kur Current
iK
K+ Channels (Delayed Rectifier), Ks, Kr, Kur Gating Mechanism
Voltage
K+ Channels (Delayed Rectifier), Ks, Kr, Kur Functional Roles
Causes phase 3 of action potential
Is enhanced by increased intracellular Ca2+
K+ Channel (ATP-Sensitive) Current
iKATP
K+ Channel (ATP-Sensitive) Gating Mechanism
Ligand
K+ Channel (ATP-Sensitive) Functional Roles
Increases K+ permeability when [ATP] is low
K+ Channel (Acetylcholine Activated) Current
iKACh
K+ Channel (Acetylcholine Activated) Gating Mechanism
Ligand
K+ Channel (Acetylcholine Activated) Functional Roles
Responsible for effects of vagal stimulation
Decreases diastolic depolarization (and the heart rate)
Hyperpolarizes resting membrane potential
Shortens phase 2 of the action potential
Na+, Ca2+, K+ (Pacemaker Current via HCN Channel) Current
if (“funny)
Na+, Ca2+, K+ (Pacemaker Current via HCN Channel) Gating Mechanism
Both
Na+, Ca2+, K+ (Pacemaker Current via HCN Channel) Functional Roles
Is activated by hyperpolarization and cyclic nucleotides and contributes to the diastolic depolarization
Is enhanced by sympathetic stimulation and B-adrenergic agents
Is suppressed by vagal stimulation
Activation and Inactivation Gates of Channels
Both gates respond to changes in membrane potential but with different voltage sensitivities and time courses
Inactivation gates of Na+ channels remain closed during the plateau phase and the remainder of the action potential, inactivating the action potential
Sustained sodium channel inactivation, combined with activation of calcium channels and delay in opening of potassium channels accounts for the long plateau phase and long cardiac refractory period, which lasts until the end of phase 3
With repolarization, both gates of the sodium channel return to their original position and the channel is ready to be reactivated by a subsequent depolarization
Factors other than Membrane Voltage that Influence Membrane Ionic Permeability and Operation of Ion Channels
High intracellular Ca2+ concentration during systole contributes to activation of certain K+ channels and increases the rate of repolarization
Sympathetic and parasympathetic input can influence some voltage-gated channels and cause activation or suppression of ligand-gated channels
Mechano-gated and mechano-modulated channels may be activated by myocyte stretch or myocyte volume changes
Depolarization Beyond Rising Phase of the Action Potential in Fast-Response Action Potential vs Slow-Response Action Potential
Slow-response action potential differs from the fast-response action potential primarily because of the lack of a strong activation of the fast Na+ channel at its onset
Depolarization beyond threshold during the rising phase of the action potential in "pacemaker" cells is slow and primarily caused by the influx of Ca2+ through slow L-type channels
How is rapid depolarization vs slow depolarization initiated in cardiac cells?
All cardiac cells are capable of having fast-type or slow-type action potentials
Rapid depolarization to threshold potential is usually forced on a cell by the occurrence of an action potential in an adjacent cell
Slow depolarization to threshold occurs when a cell itself spontaneously and gradually loses its resting polarization, which usually happens only in the SA or AV node
How do cardiac muscle cells connect?
Cardiac muscle cells branch and connect end-to-end with neighboring cells in cells called intercalated disks which contain
Firm mechanical attachments between adjacent cell membranes by adherins in desmosomes
Low-resistance electrical connections between adjacent cells through channels formed by connexin in gap junctions