T6:The rate and extent of chemical change.
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
What is the equation linking the quantity of reactant used,mean rate of reaction and time taken?
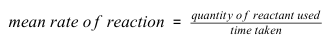
What is the equation linking the quantity of product formed,mean rate of reaction and time taken?
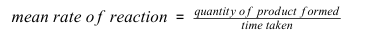
How can the quantity of a reactant or product be measured by?
Mass in grams or by a volume in cm3.
What are the two units for the rate of a reaction?
g/s or cm3/s.
How does increasing the temperature increase the rate of reaction?
When the temperature of the particles in increased
they gain more kinetic energy
so move faster
so they collide more frequently
Therefore,the faster they move,the more collision they have so more of the particles will have the activitation energy to amke the reaction happen.
How does increasing the concentration increase the rate of reaction?
If the concentration is increased then there are more particles in the same volume
meaning that there are more freuent collisions
nand more collisions between particles with the activation energy
means that it increases the rate of reaction
How does increasing the pressure increase the rate of reaction?
Increasing the pressure
means that there is less sapce between the particles
so there are more frequent collisions between the particles
leading to a higher probability of successful reactions
increasing the rate of the reaction
How does increasing the surface area increase the rate of reaction?
Increasing the surface area
means tht for the same volume of solid
the particles will have more area to work on
so there wil be more frequent collisions
increasing the rate of reaction
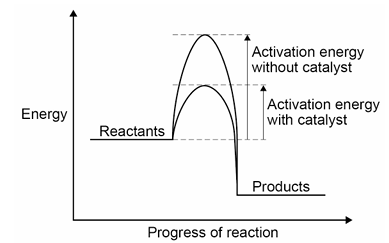
How does a catylst increase the rate of reaction?
A catalyst increasing the arte of reaction
by decreasing the activation energy needed for the reaction to occur
which is done by the catalyst providing an
alternate reaction pathway with a lower activation energy.
When is the only instance when chemical reactions can occur?
Chemical reactions can occur only when reacting particles collide with each other and with sufficient energy.
What is the activation energy?
The minimum amount of energy that particles must have to react.
What is a catalyst?
A catalyst is a substance that increases the rate of a reaction wihtout being used up in the reaction itself.
What do different reactions need?
Different catalysts.
What do enzymes act as in bioloicl systems?
Catalysts.
Draw a reaction profile for a catalysed reaction: