VSEPR + Electronegativity
1/23
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
Nonpolar covalent
0.0-0.4, between nonmetals, electrons shared EQUALLY
polar covalent
0.5-1.7, between nonmetals, electrons shared UNEQUALLY- causes dipole!
Ionic
1.8+, metal and nonmetal, electrons TRANSFERRED
electron flow of dipole
arrow points towards more electronegative element
dipole charge
MORE e-neg element is partially NEGATIVE, LESS e-neg element is partially POSITIVE
degrees of linear angle
180
degrees of trigonal planar
120
degrees of bent
120
degrees of tetrahedral
109.5
degrees of trigonal pyramidal
109.5
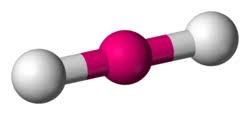
What molecular geometry
linear
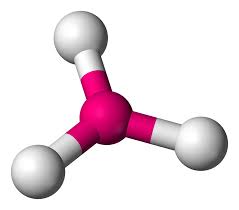
What molecular geometry
trigonal planar
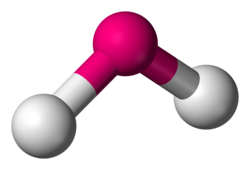
What molecular geometry
bent
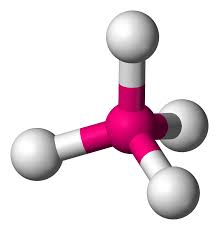
What molecular geometry
tetrahedral

What molecular geometry
trigonal pyramidal
molecular geometry vs. electron geometry
electron geometry: 3D arrangement of both bonding atoms AND lone pairs
molecular geometry: 3D arrangement of ONLY atoms- NO LONE PAIRS
for this test, we look at molecular geometry
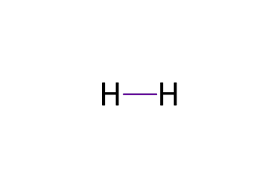
LDS for H2
LDS for CO2
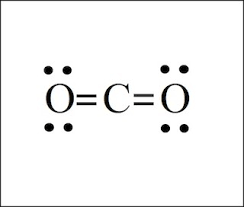
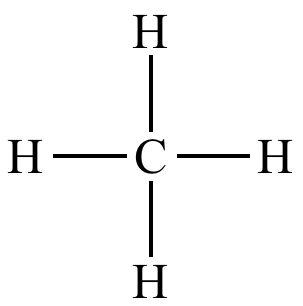
LDS for CH4
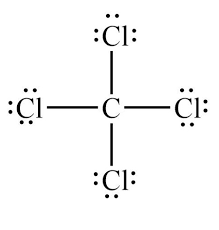
LDS for CCl4
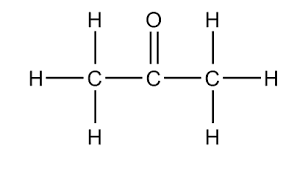
LDS for H3CCOCH3
Show ions for NaCl
Na+Cl-
Show ions for HF
H+F-
Show ions for RbO
Rb+O-