CHEM-HARD ORGANIC CHEM QUESTIONS
1/47
Earn XP
Description and Tags
HARD ORGANIC CHEM QUESTIONS
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms

WHAT IS THE ANSWER TO THIS QUESTION

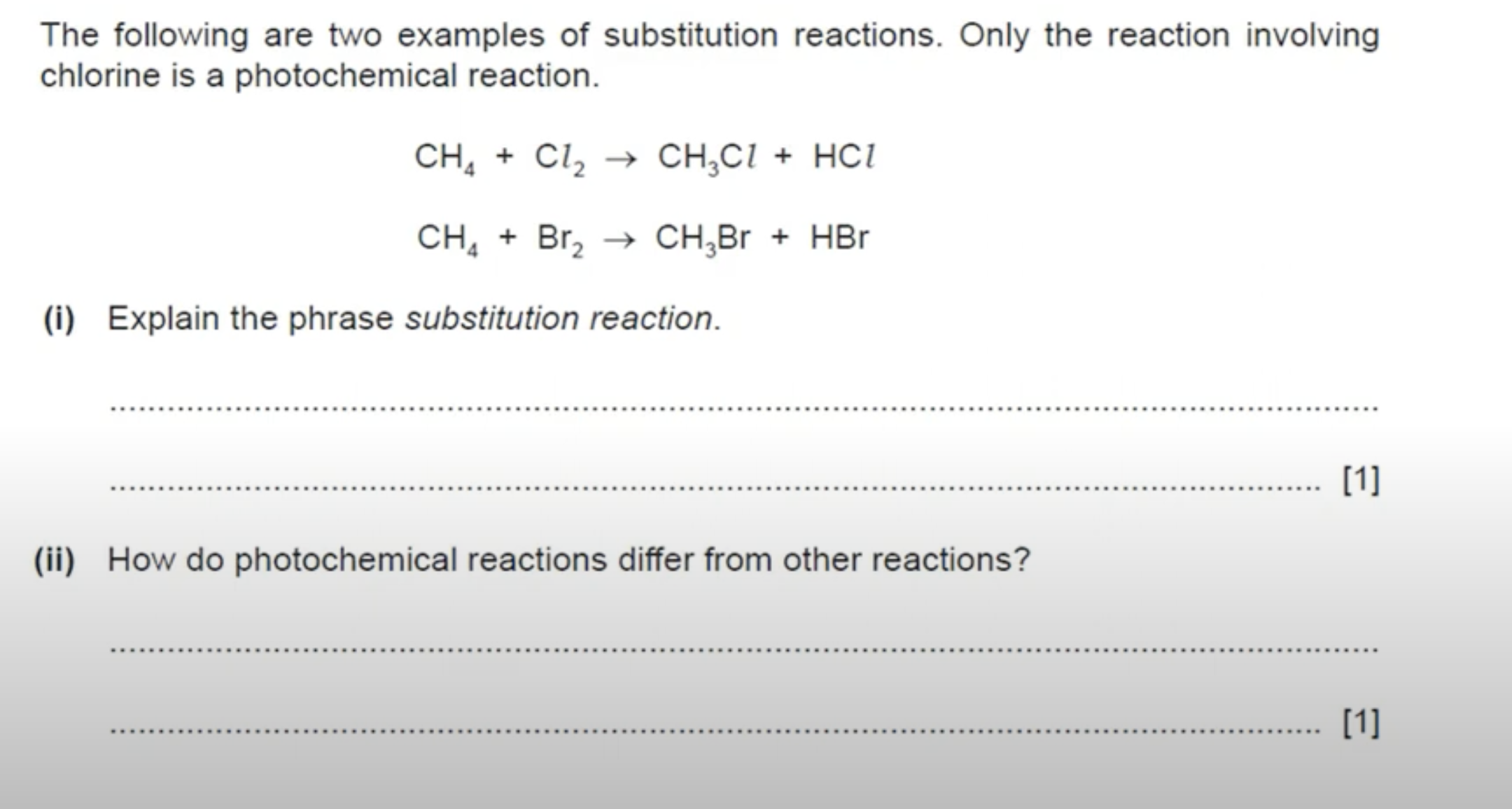
WHAT IS THE ANSWER TO THIS QUESTION
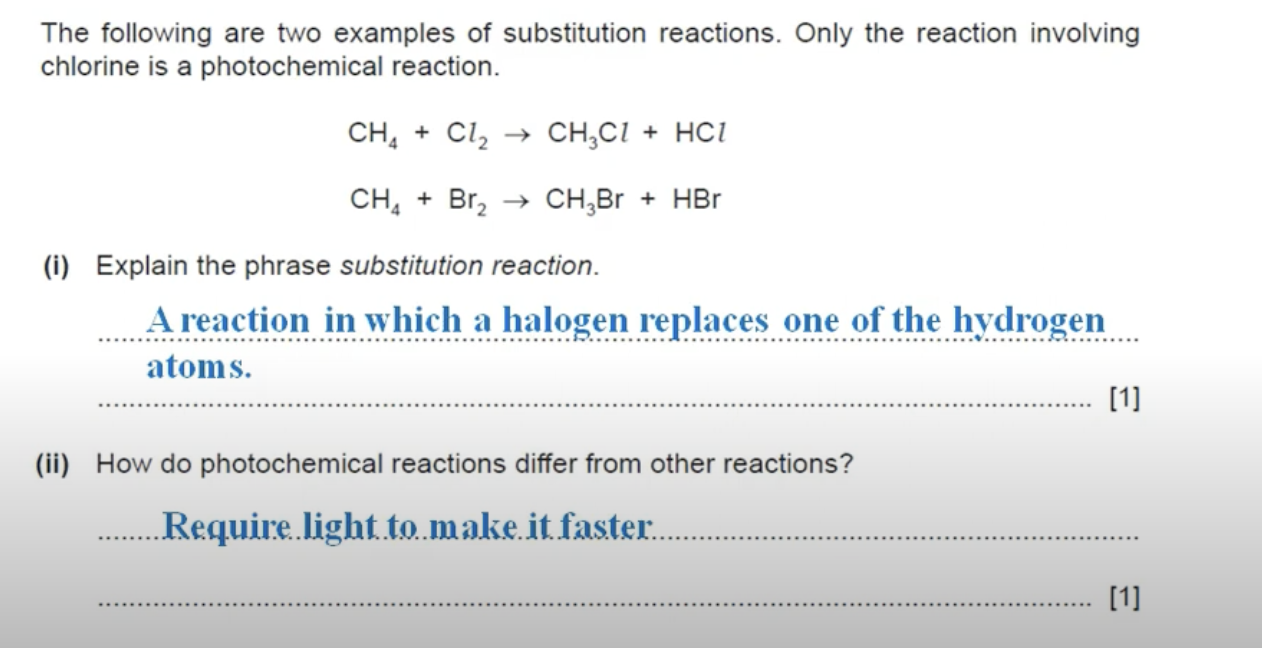
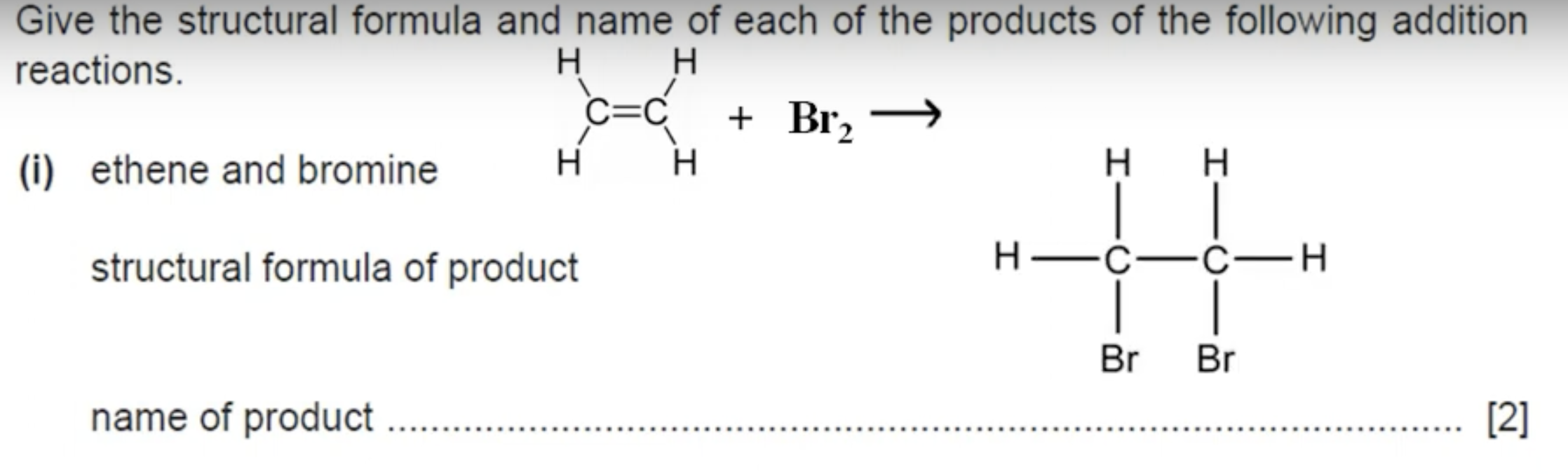
WHAT IS THE ANSWER TO THIS QUESTION
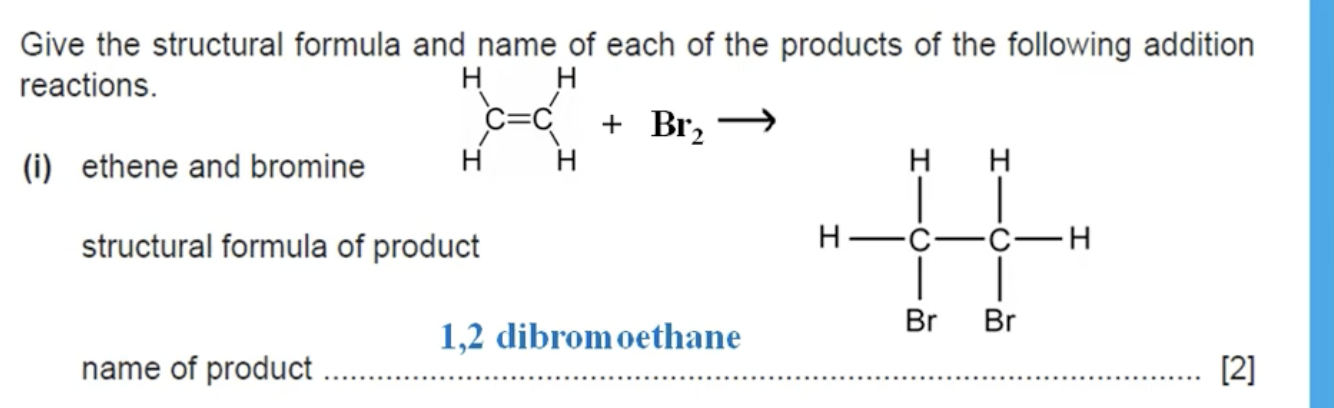
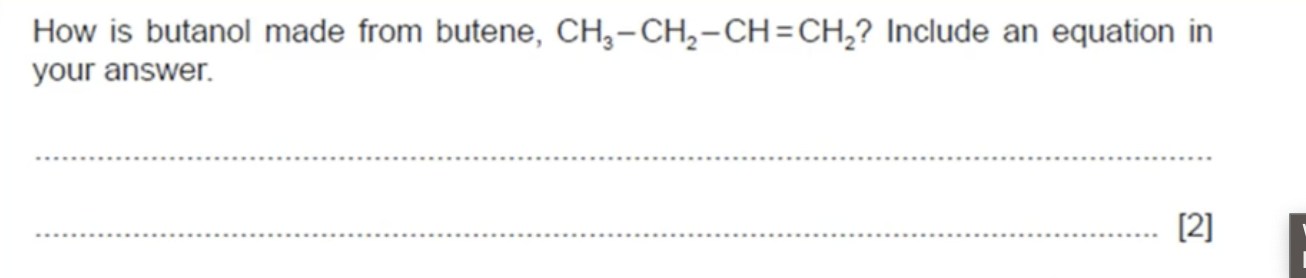
WHAT IS THE ANSWER TO THIS QUESTION
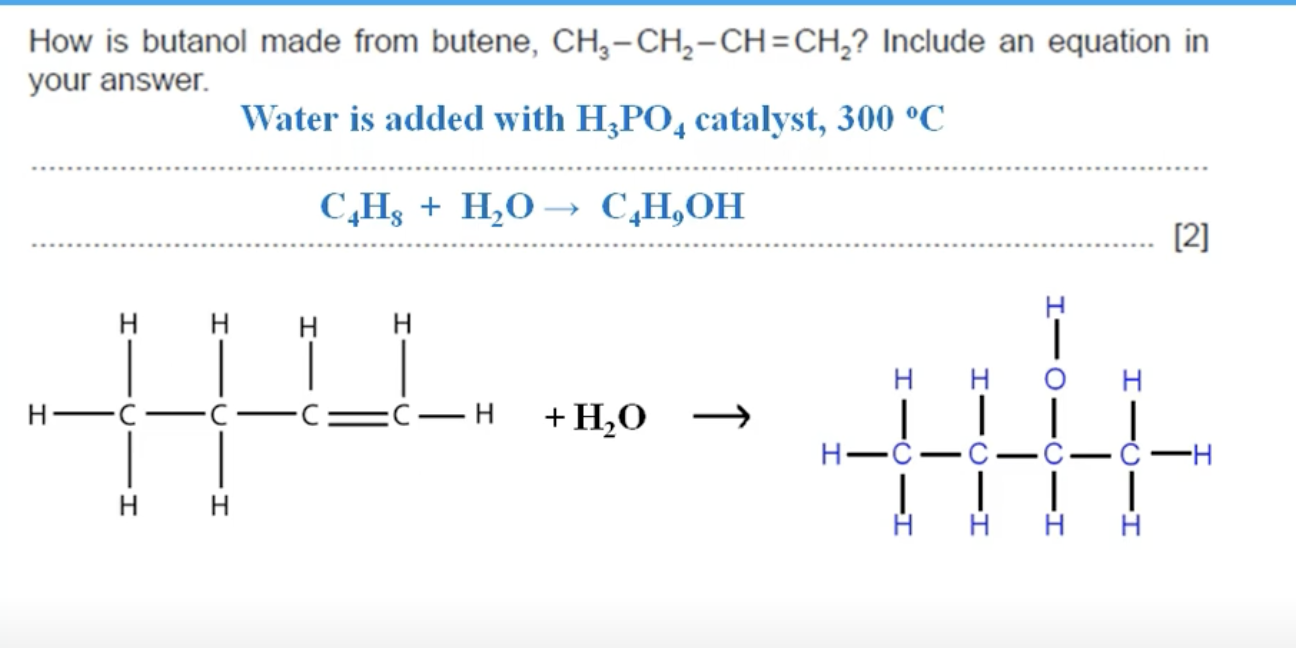
WHAT IS THE ANSWER TO THIS QUESTION
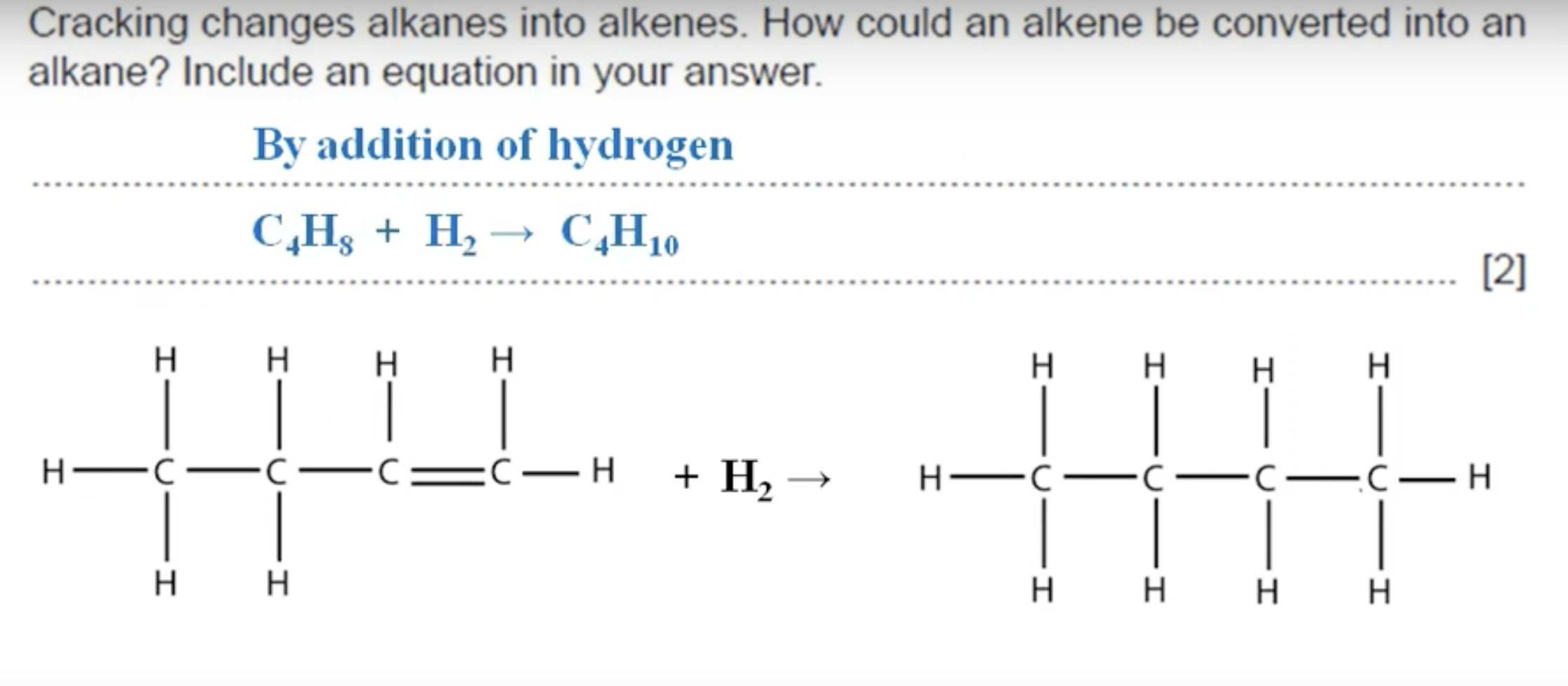

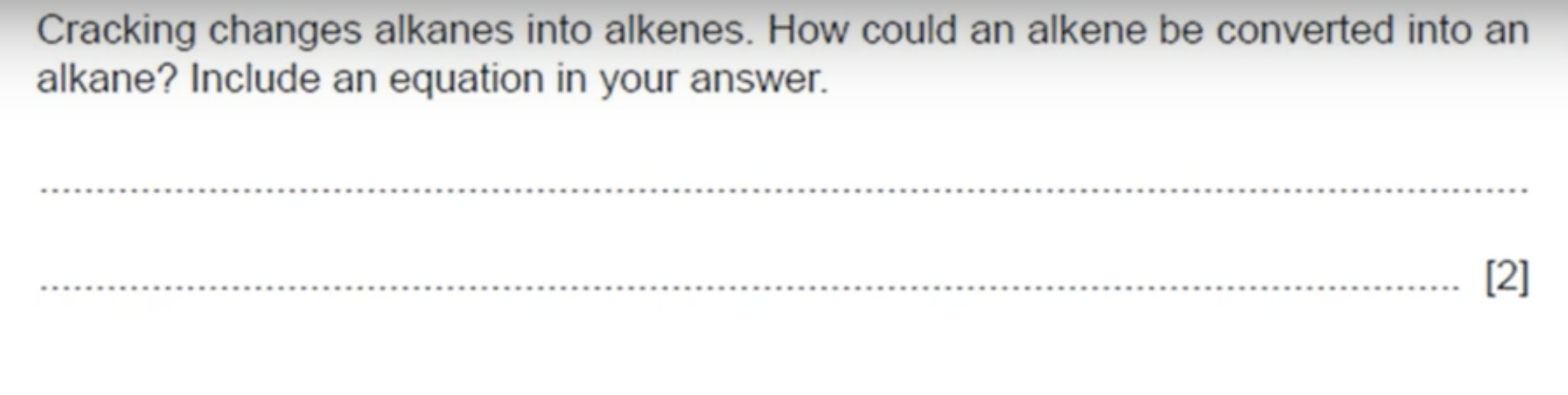
WHAT IS THE ANSWER TO THIS QUESTION


WHAT IS THE ANSWER TO THIS QUESTION
THE ALKENE BREAKS AT THE DOUBLE BOND TO GIVE AN ACID ON BOTH SIDES. THUS FOR THE ALKENE PENTENE(FIRST ONE WITH 5 CARBON ATOMS) BREAKS UP AT THE DOUBLE BOND. AS YOU CAN SEE THERE ARE 2 CARBONS(ETH) ON THE LEFT SIDE OF THE DOUBLE BOND WHICH WILL GIVE ETHANOIC ACID AND THERE ARE 3 CARBONS(PROP) ON THE RIGHT SIDE OF THE DOUBLE BOND WHICH WILL GIVE PROPANOIC ACID WHEN THE DOUBLE BOND BREAKS.
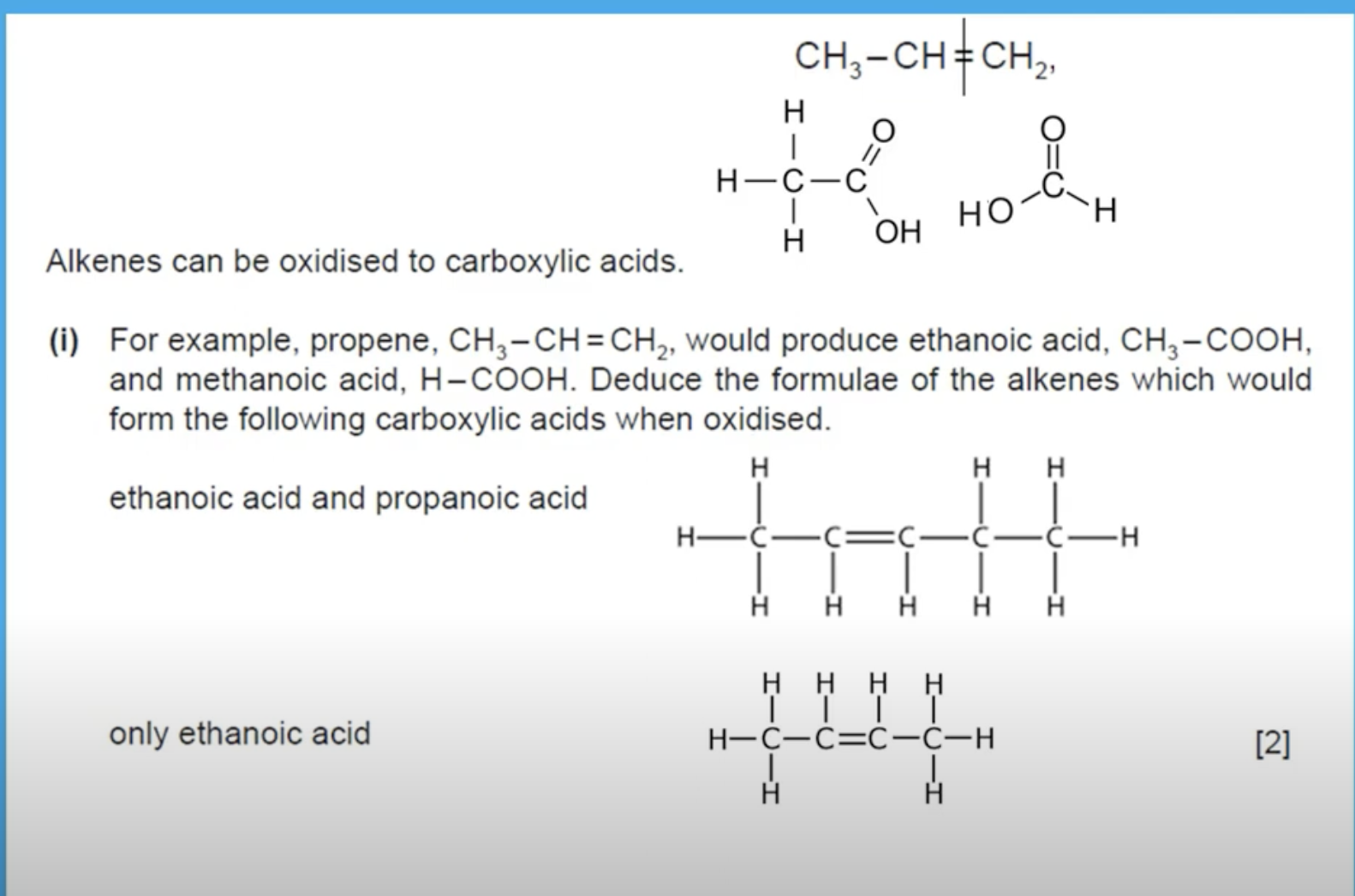
CAN ALKENES BE OXIDISED TO FORM CARBOXYLIC ACIDS.
YES, ALKENES CAN BE OXIDISED TO FORM CARBOXYLIC ACID
WHAT IS THE ANSWER TO THIS QUESTION
Acetobacter bacteria: This is the primary bacteria used in the aerobic fermentation process. These bacteria are capable of oxidizing ethanol to acetic acid in the presence of oxygen.
The process is often referred to as vinegar fermentation, because it is the method used to produce vinegar (which contains acetic acid).

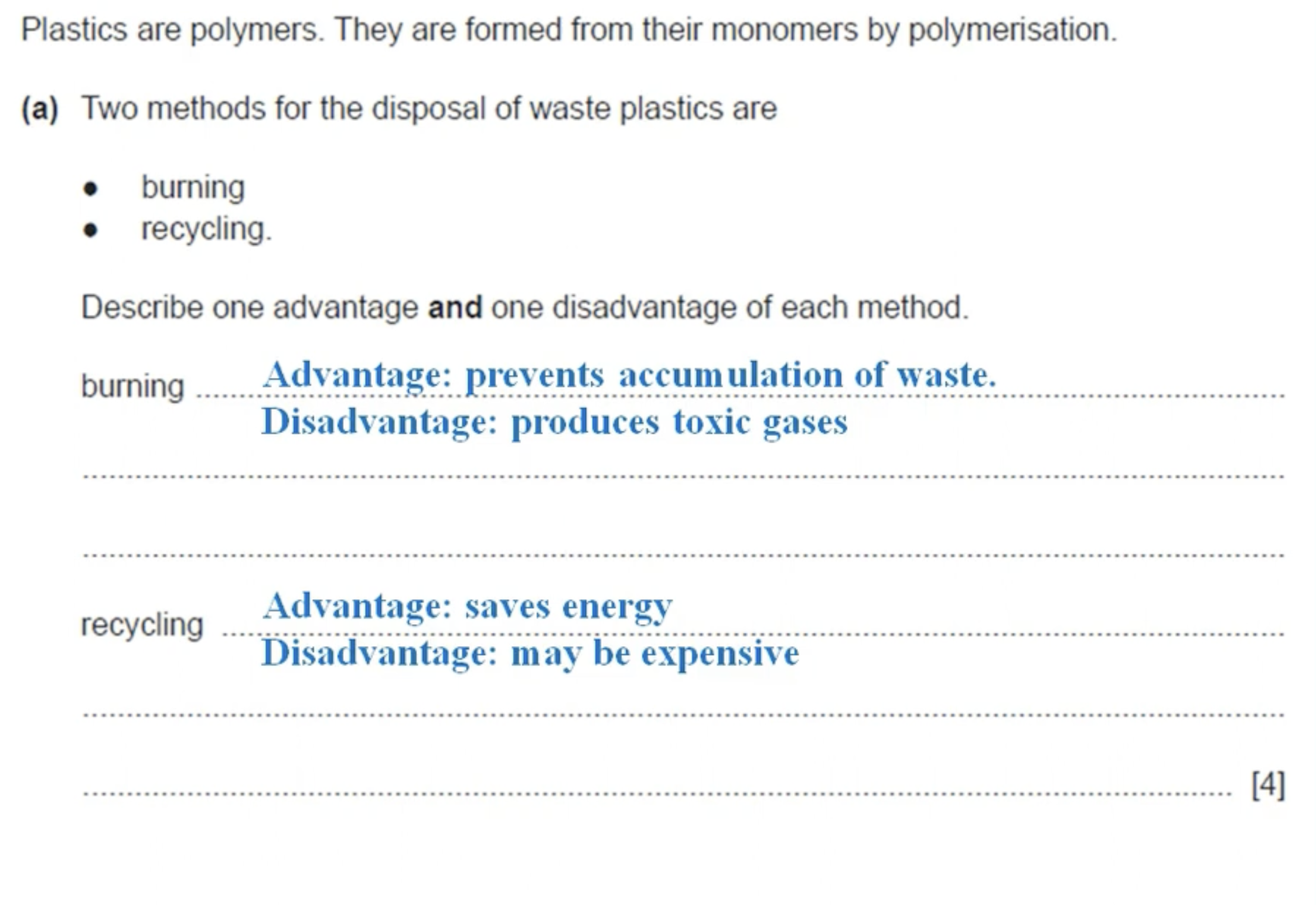
WHAT IS THE ANSWER TO THIS QUESTION
WHAT IS PVA POLYMER USED IN
IT IS USED IN PAINTS AND ADHESIVES.
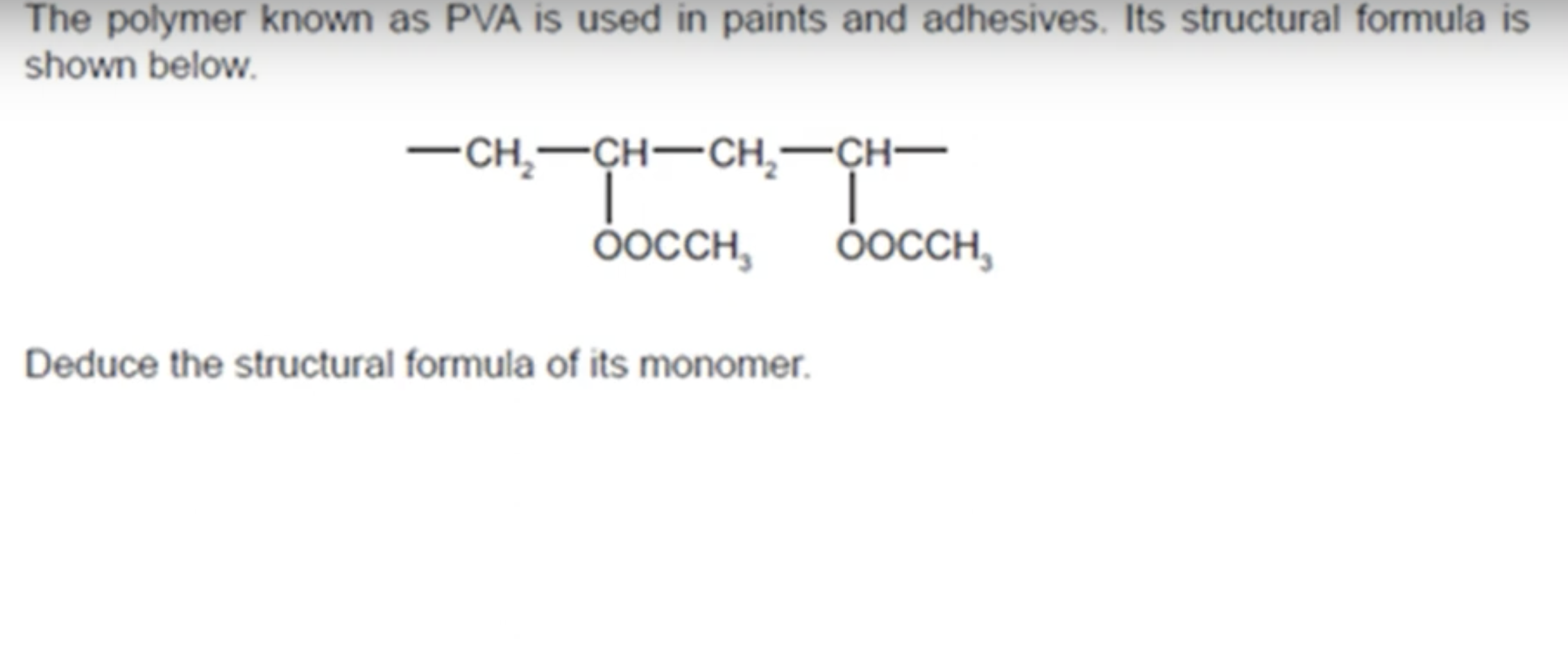
WHAT IS THE ANSWER TO THIS QUESTION
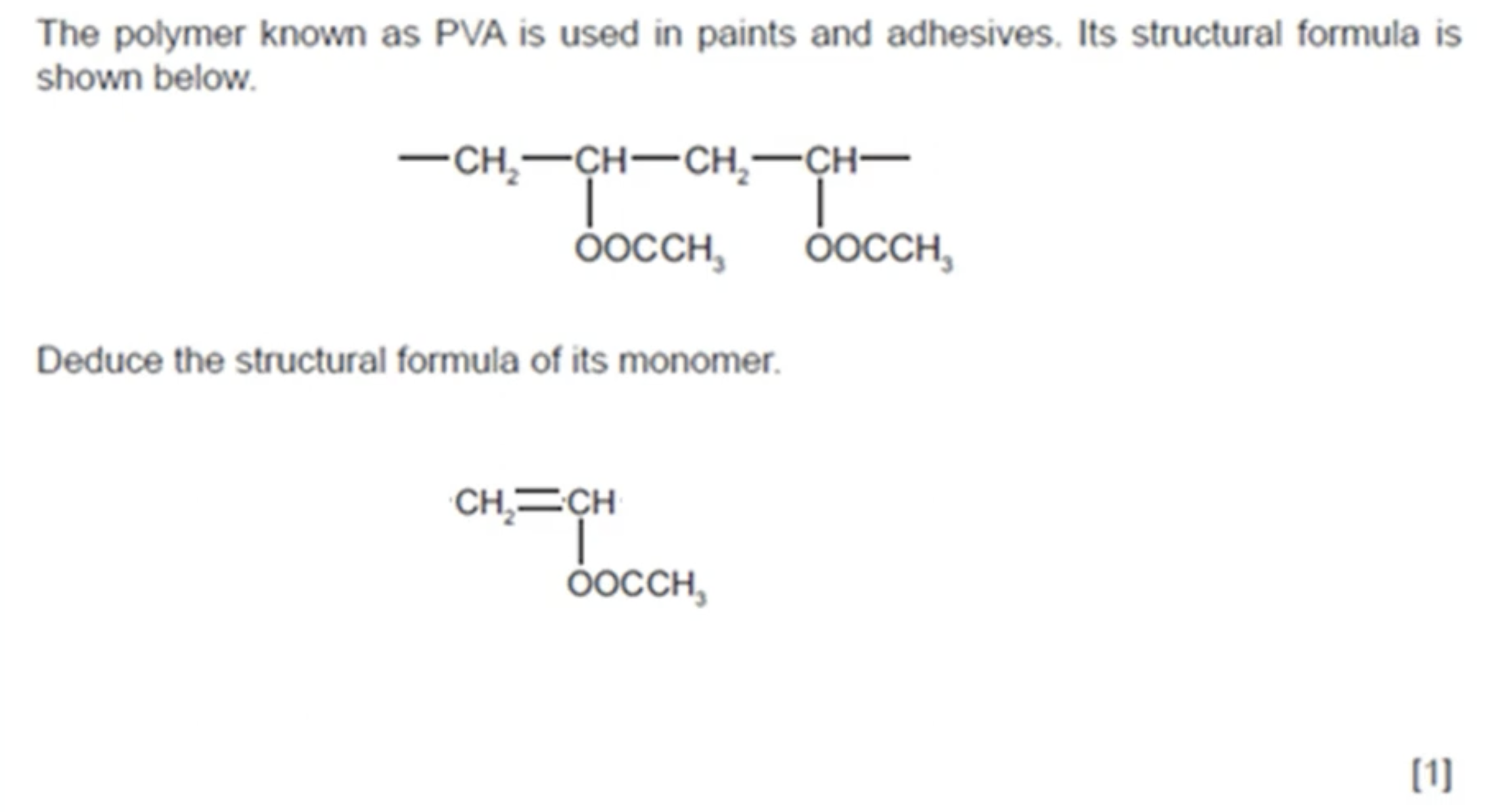
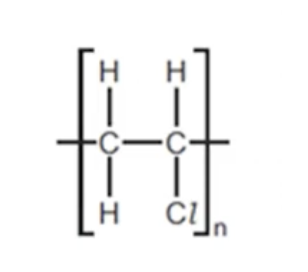
WHAT IS THE POLYMER PVC USED FOR AND WHAT PROPERTIES DOES PVC HAVE THAT MAKES IT SUITABLE FOR THAT USE
IT IS MAINLY USED FOR INSULATION OF ELECTRIC CABLES. IT IS SUITABLE FOR THIS BECAUSE:-
IT IS A POOR CONDUCTOR OF ELECTRICITY
DOES NOT CORRODE
WHAT ARE TWO POISONOUS GASES YOU GET DURING THE COMBUSTION OF PVC
YOU GET:-
CARBON MONOXIDE
CHLORINE
NOTE: THE PHOTO SHOWS THE MONOMER THAT MAKES UP THE POLYMER PVC. AS YOU CAN SEE IT CONTAINS A CHLORINE ATOM. USING THIS INFORMATION WE CAN DEDUCE THAT IT WILL ALSO RELEASE CHLORINE GAS DURING COMBUSTION.
WHAT IS THE ANSWER TO THIS QUESTION
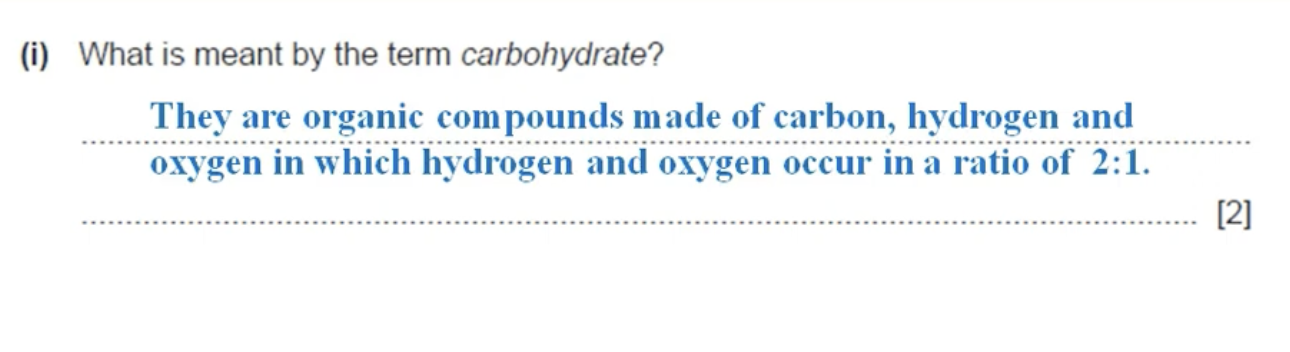
WHAT IS THE ANSWER TO THIS QUESTION
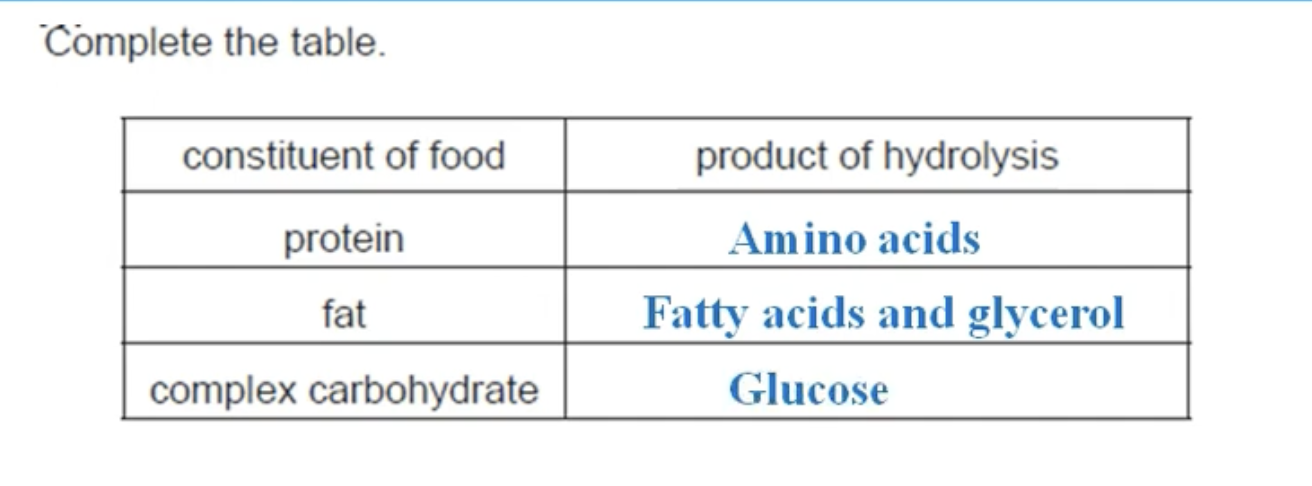

WHAT IS THE ANSWER TO THIS QUESTION
FATS ARE POLYESTERS
PROTEINS ARE POLYAMIDES


WHAT IS THE ANSWER TO THIS QUESTION
Acid-catalyzed hydrolysis (using HCl or other acids) involves the addition of water molecules, but the acid helps speed up the process by donating protons (H⁺), which make the carbohydrate bonds easier to break.
The water molecules then participate in breaking the bonds between sugar units (like in starch or disaccharides), resulting in the formation of simpler molecules (monosaccharides like glucose).
PROTEIN OR COMPLEX POLYMERS CAN BE HYDROLYSED INTO SMALLER MONOMERS BY USING ENZYMES ALSO.
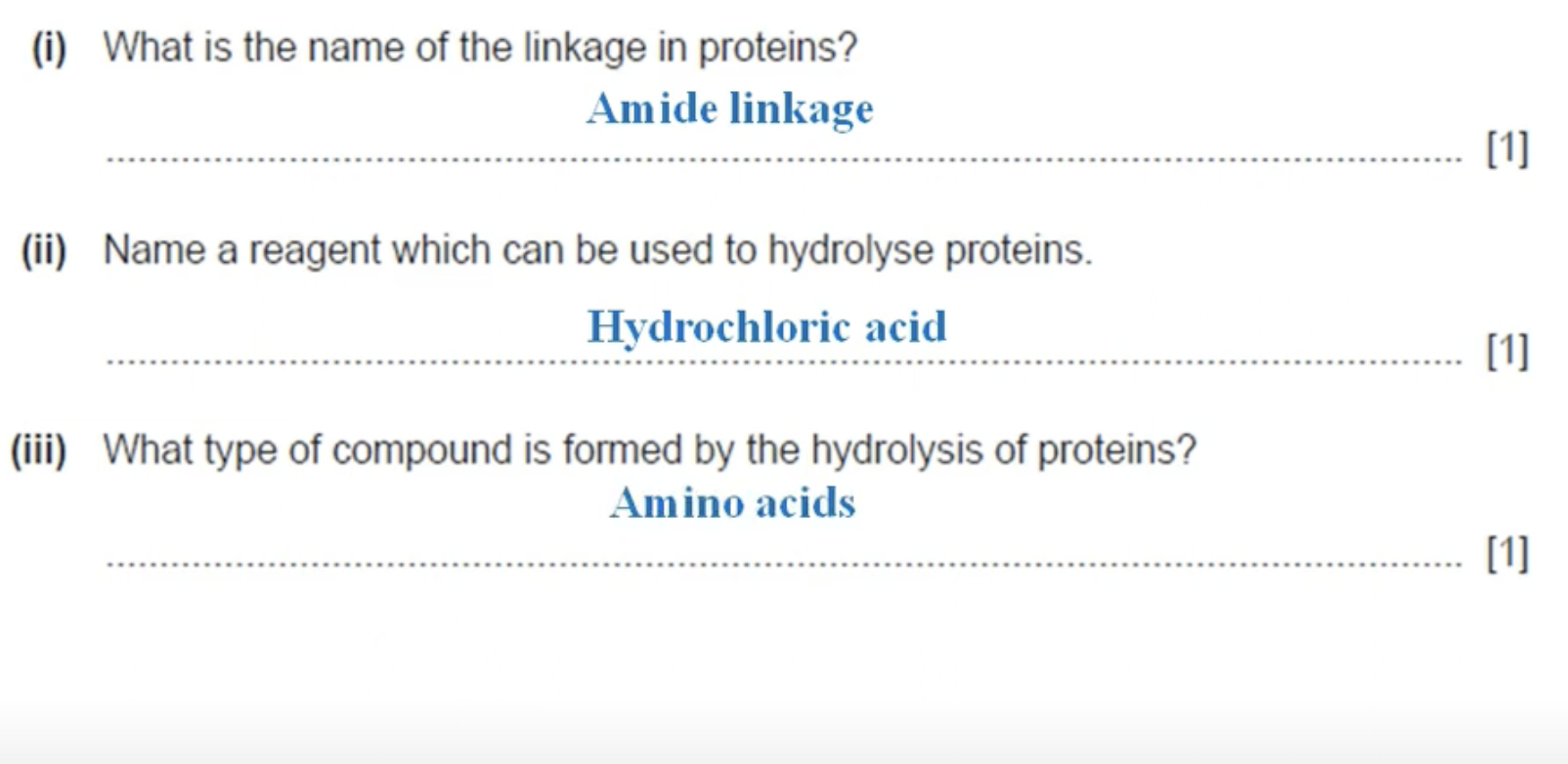
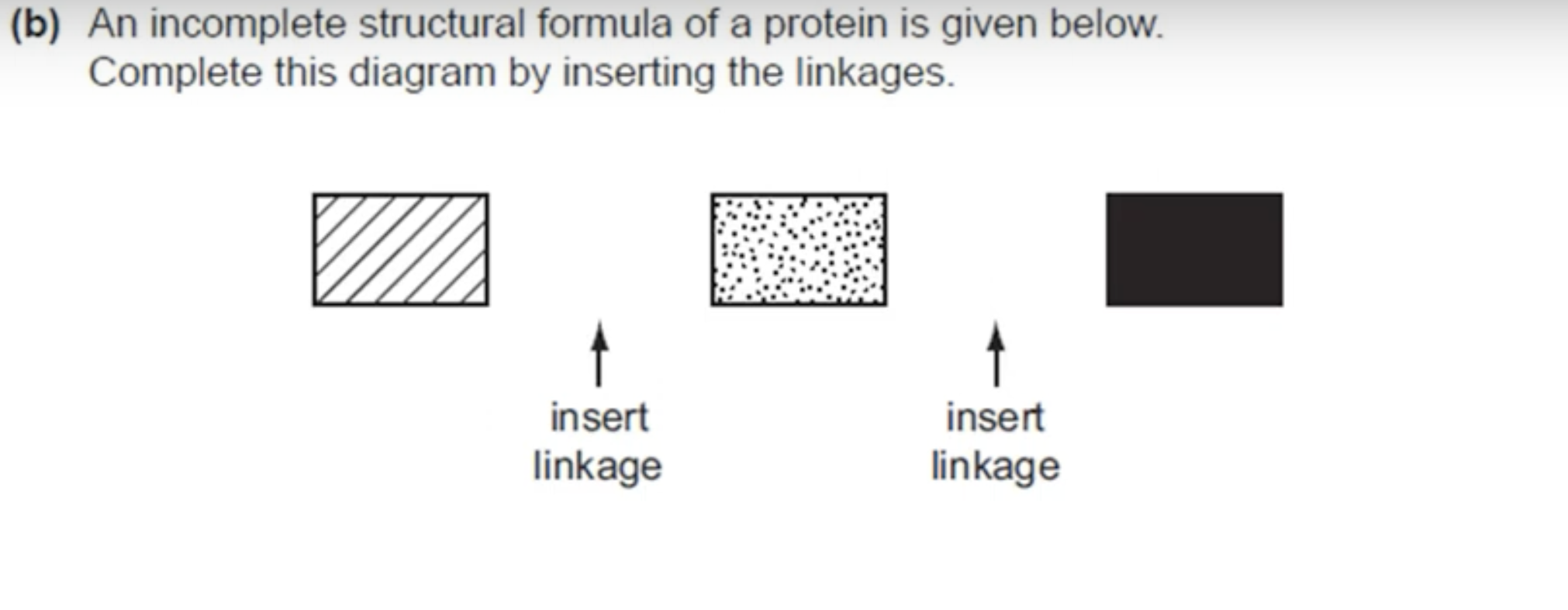
WHAT IS THE ANSWER FOR THIS QUESTION
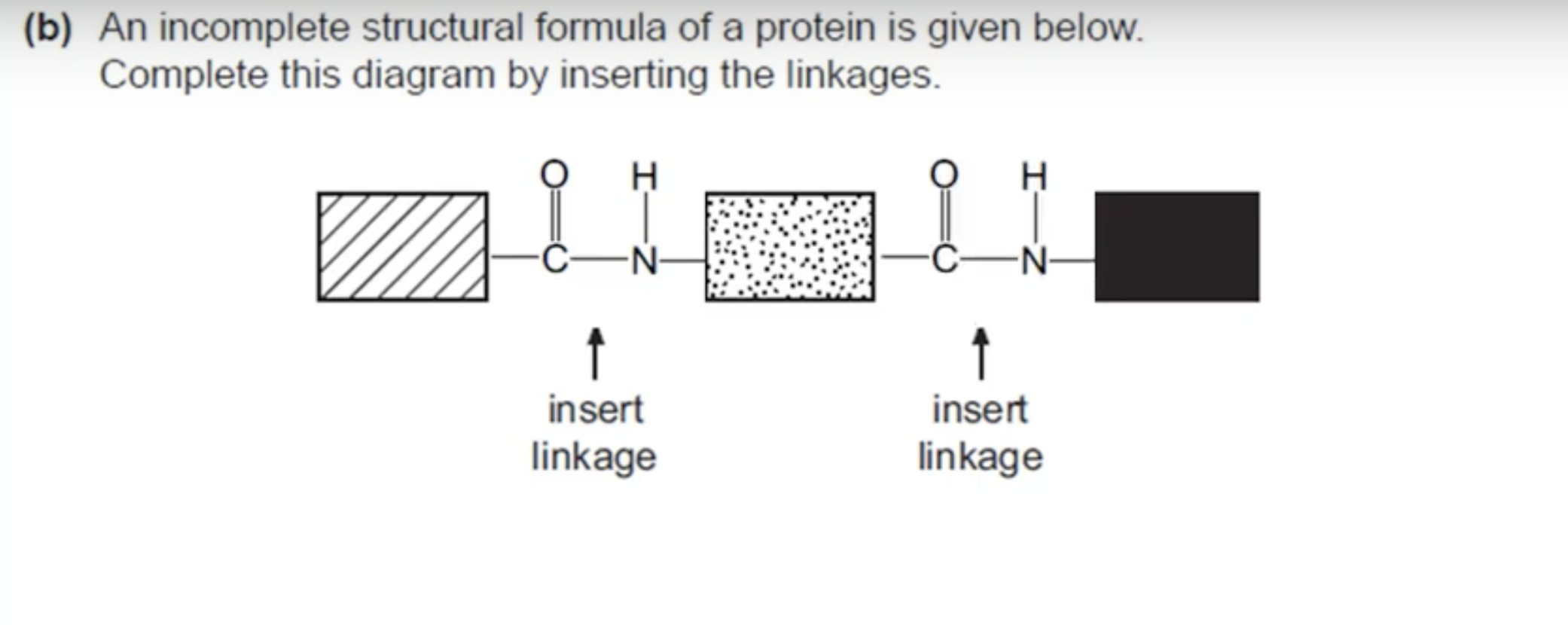

WHAT IS THE ANSWER FOR THIS QUESTION
Put 10cm3 of sunflower oil in a test tube. Put 10cm3 of ethanol in the same test tube with the sunflower oil. Ethanol is used as oils cannot dissolve in water and can only dissolve in organic solvents like ethanol.
Pour bromine water drop by drop using a dropper, and count the number of drops it takes until the orange changes to colourless.
Repeat the same steps with 10cm3 of all the other oils.
The oil that takes the most amount of bromine water drops to change colour from orange to colourless is the one that has the largest number of C=C bonds.
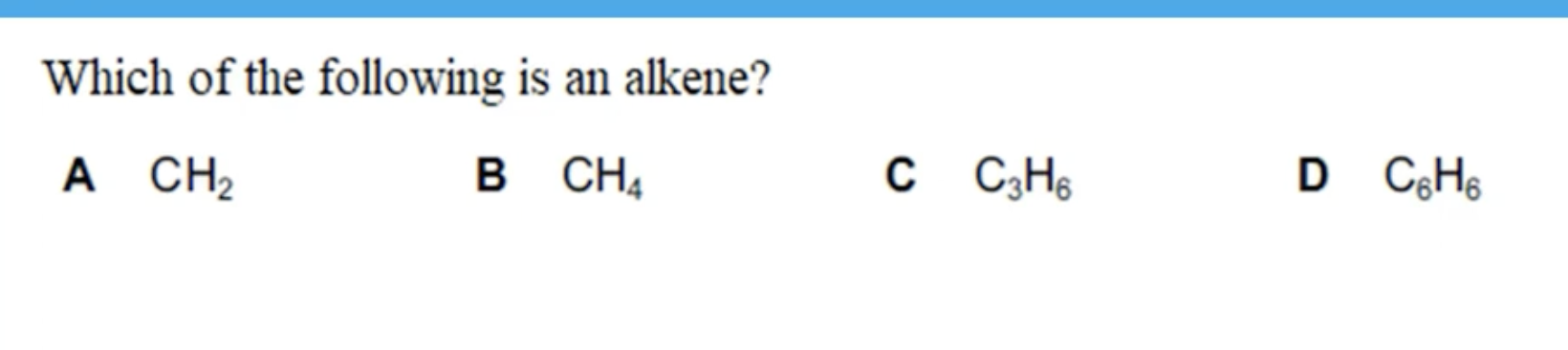
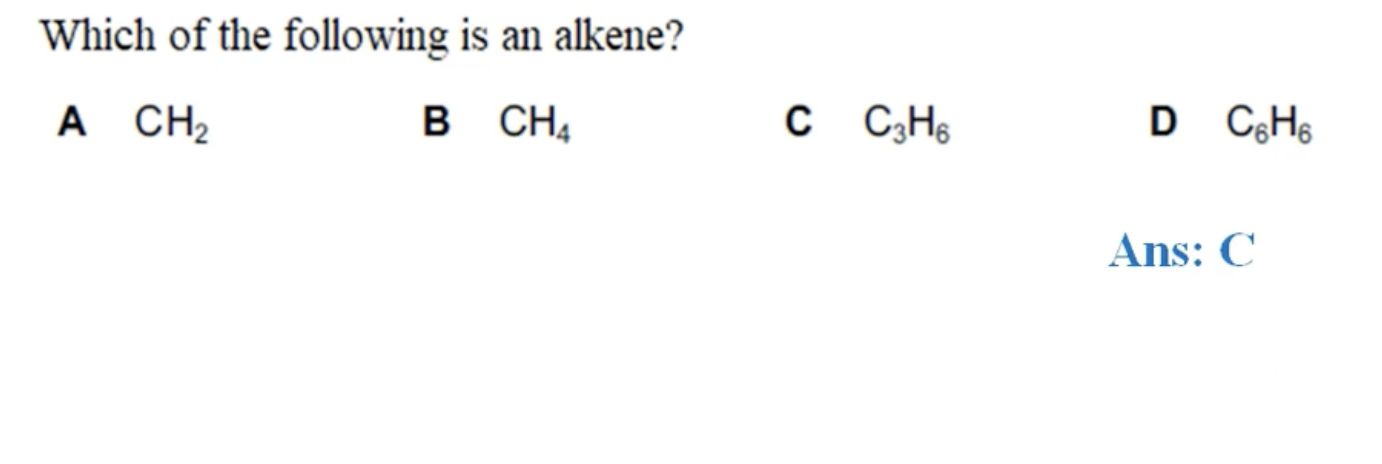
WHAT IS THE ANSWER FOR THIS QUESTION
IT WONT BE CH2 AS YOU NEED TO HAVE AT LEAST 2 CARBON ATOMS TO HAVE A DOUBLE BOND.
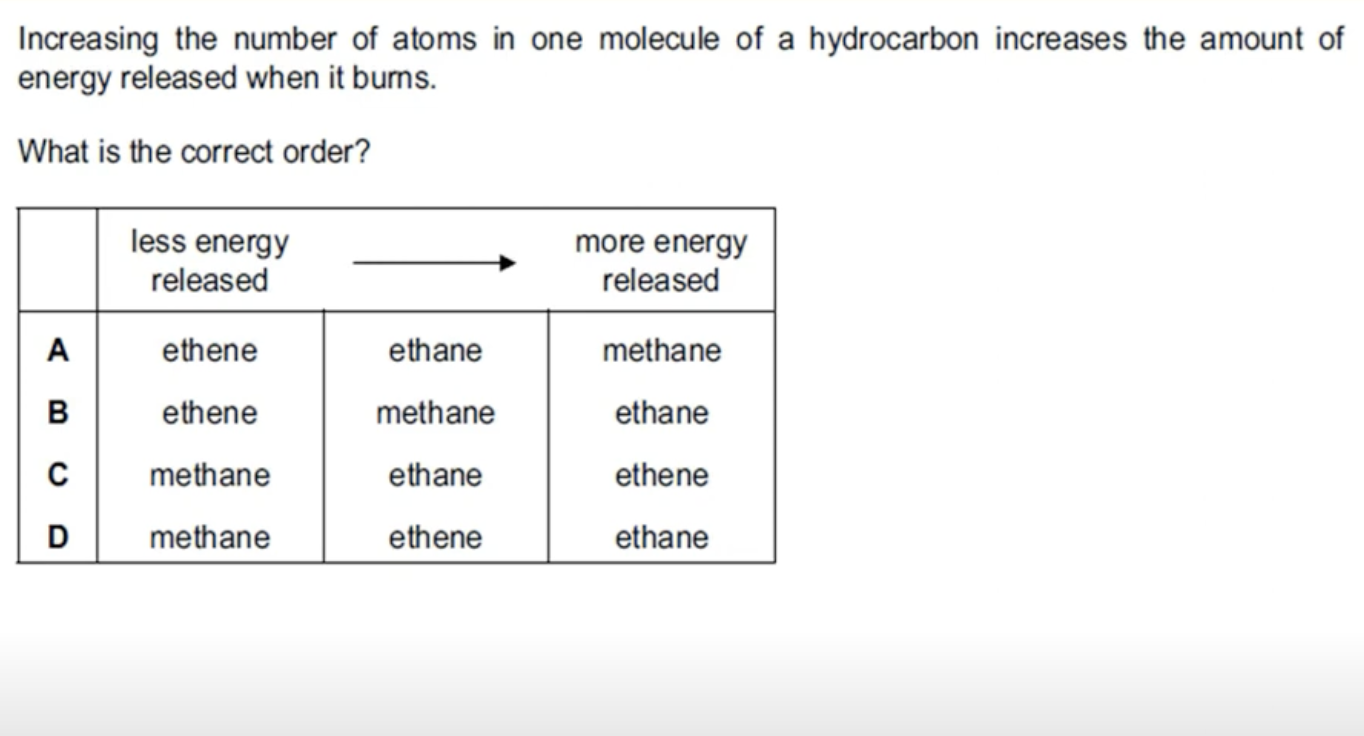
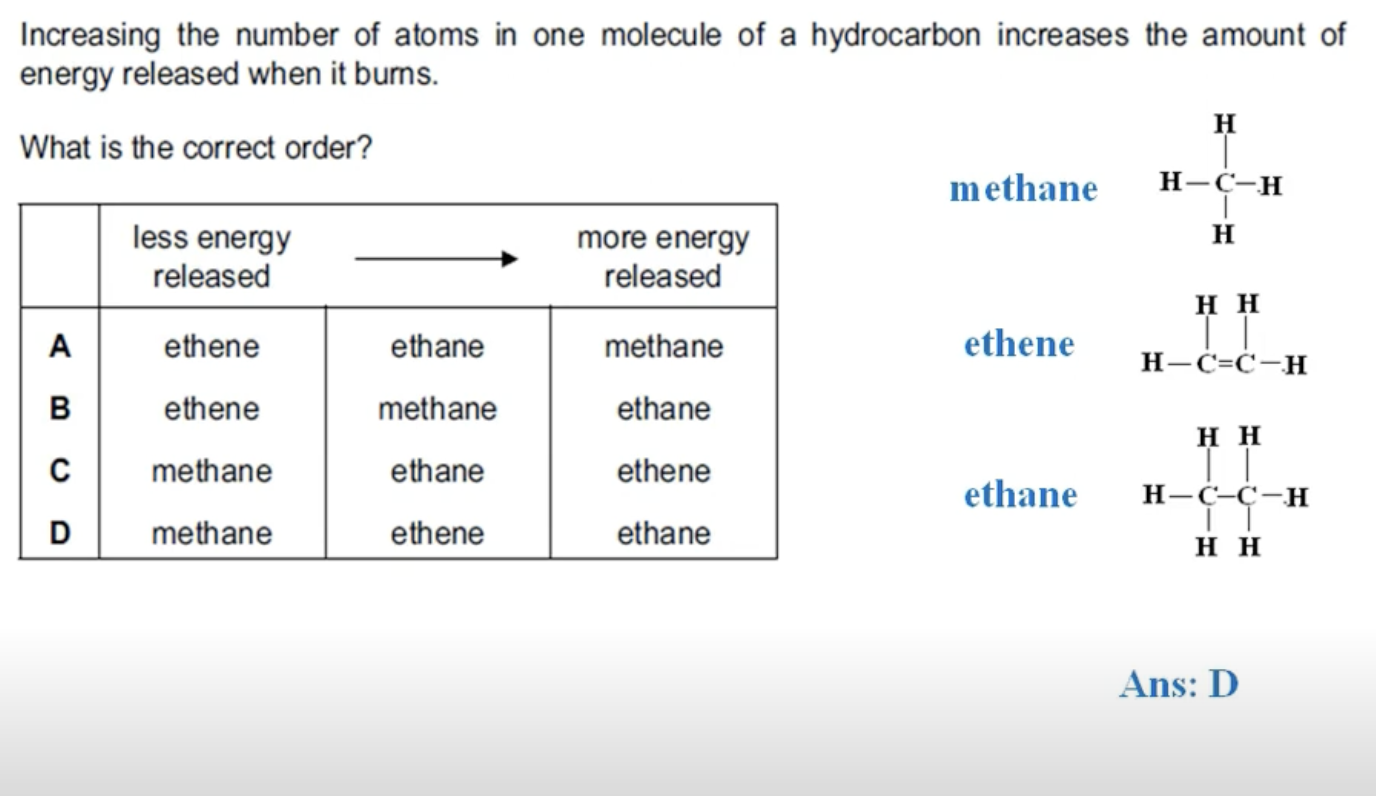
WHAT IS THE ANSWER FOR THIS QUESTION
ETHANE IS AN ALKANE AND HAS TWO MORE HYDROGEN ATOMS THAN ETHENE, THUS IT HAS MORE NUMBER OF ATOMS THAT ETHENE AND RELEASES MORE ENERGY WHEN IT IS BURNED.
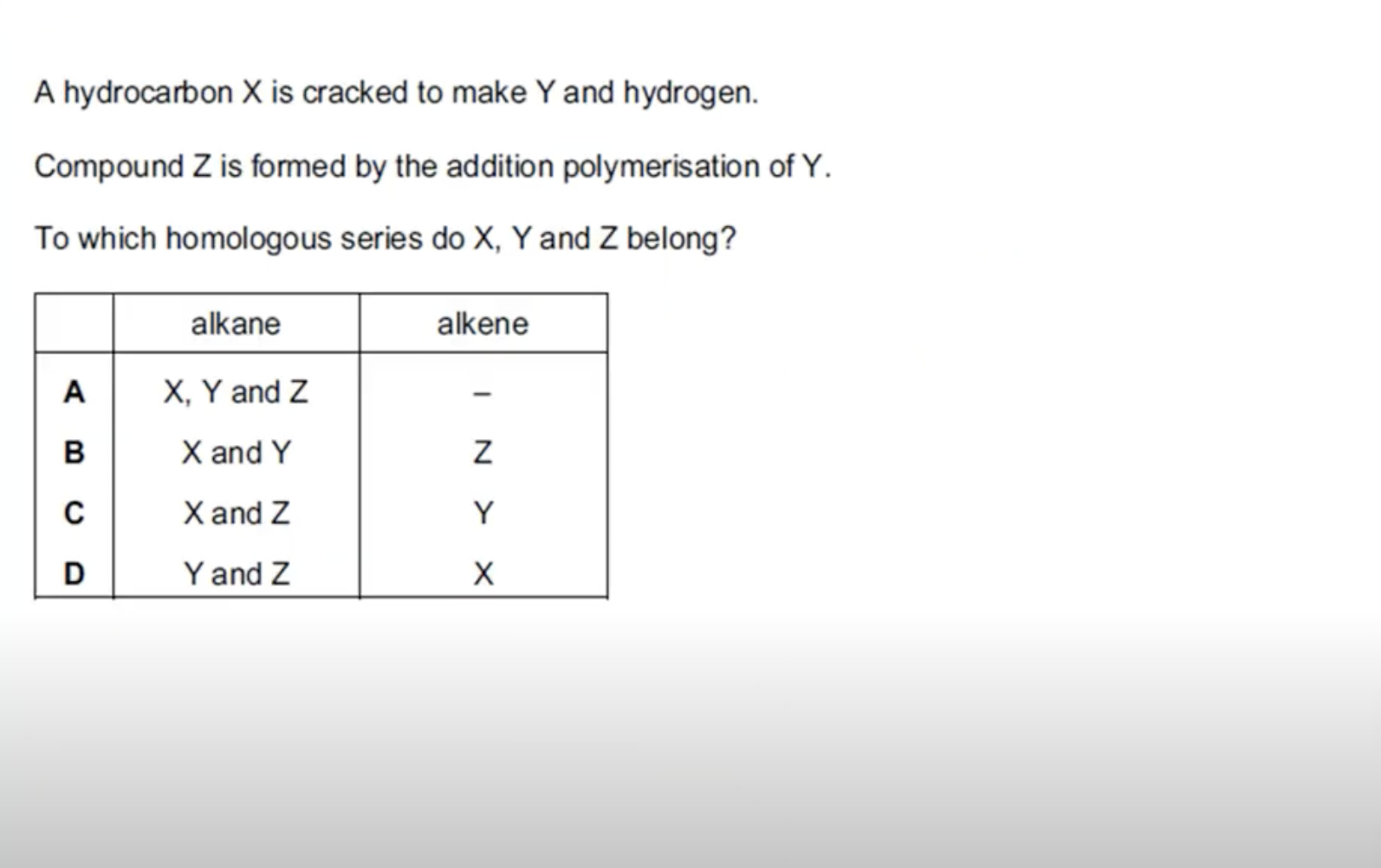
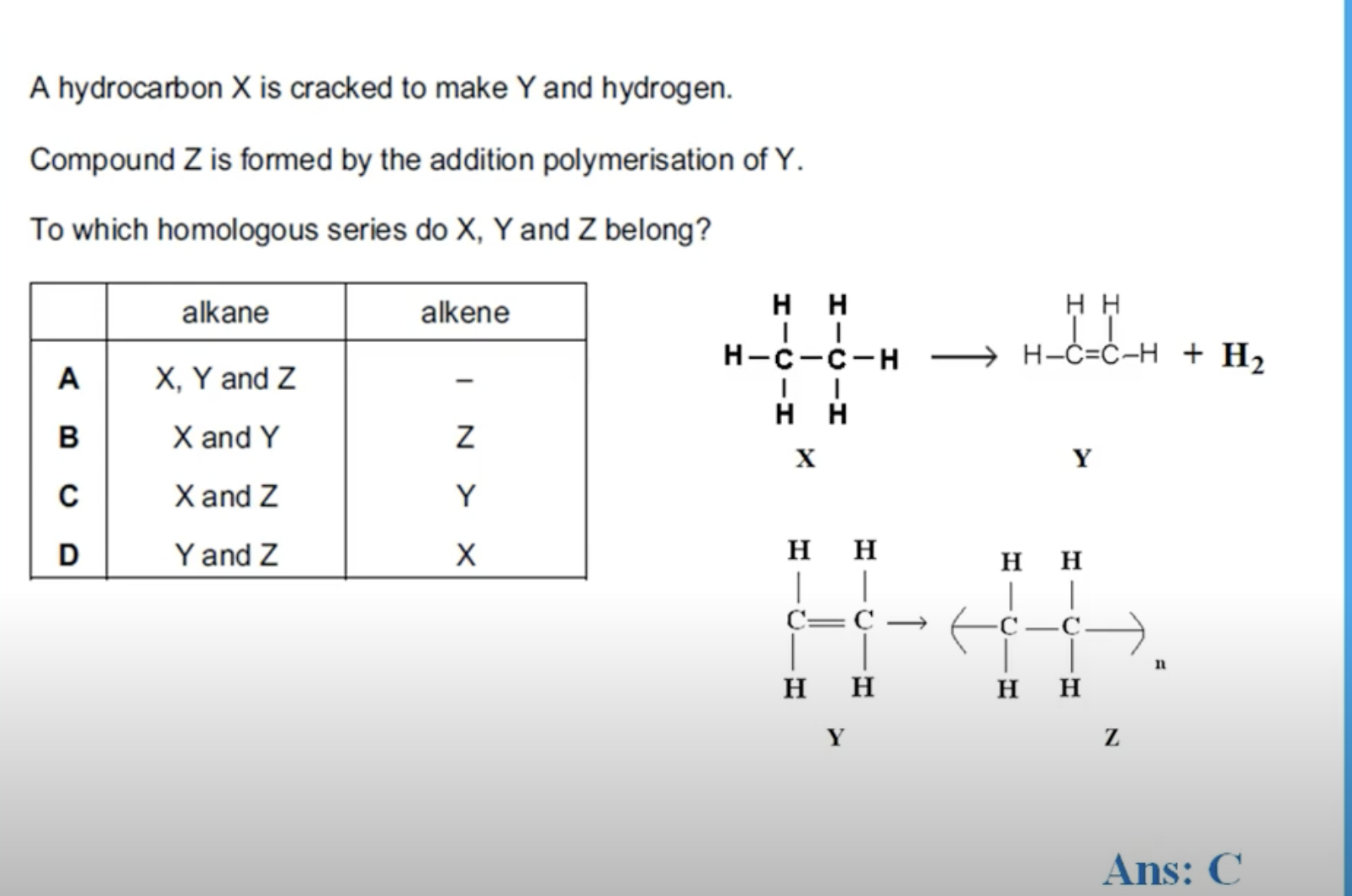
WHAT IS THE ANSWER FOR THIS QUESTION
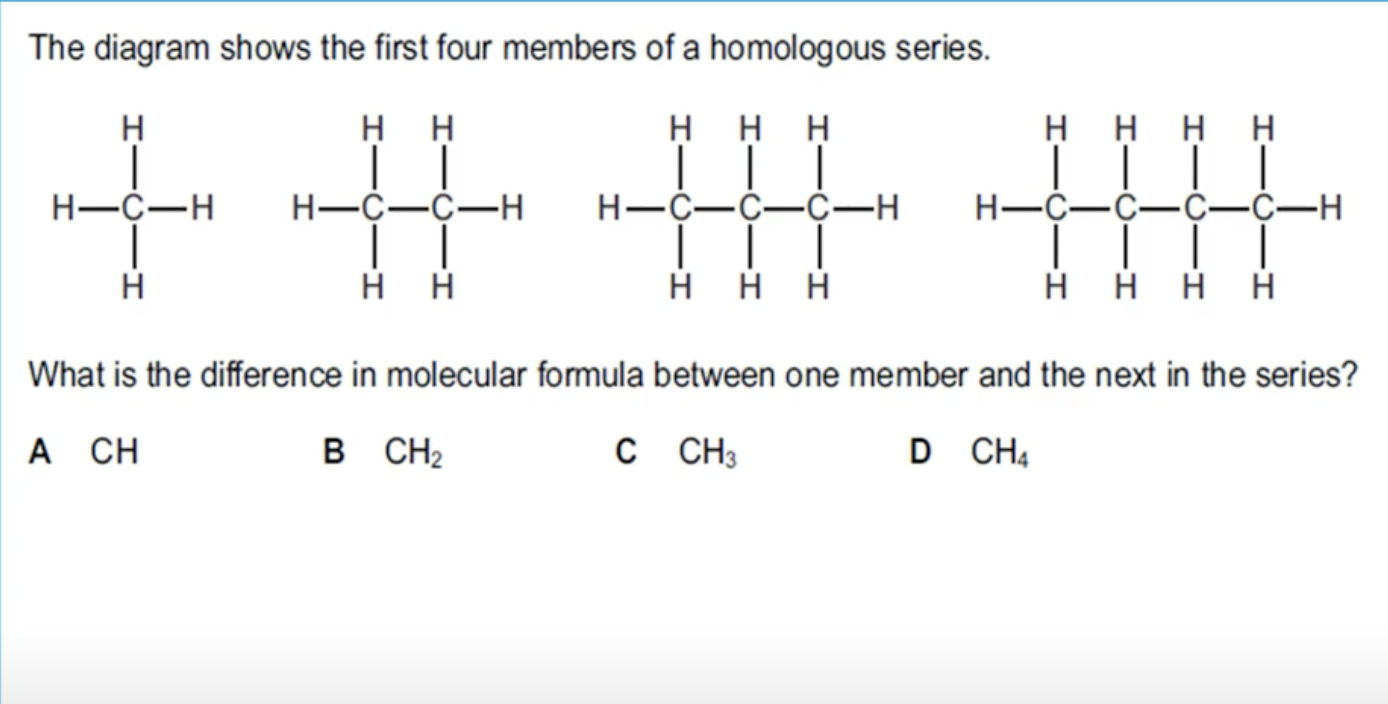
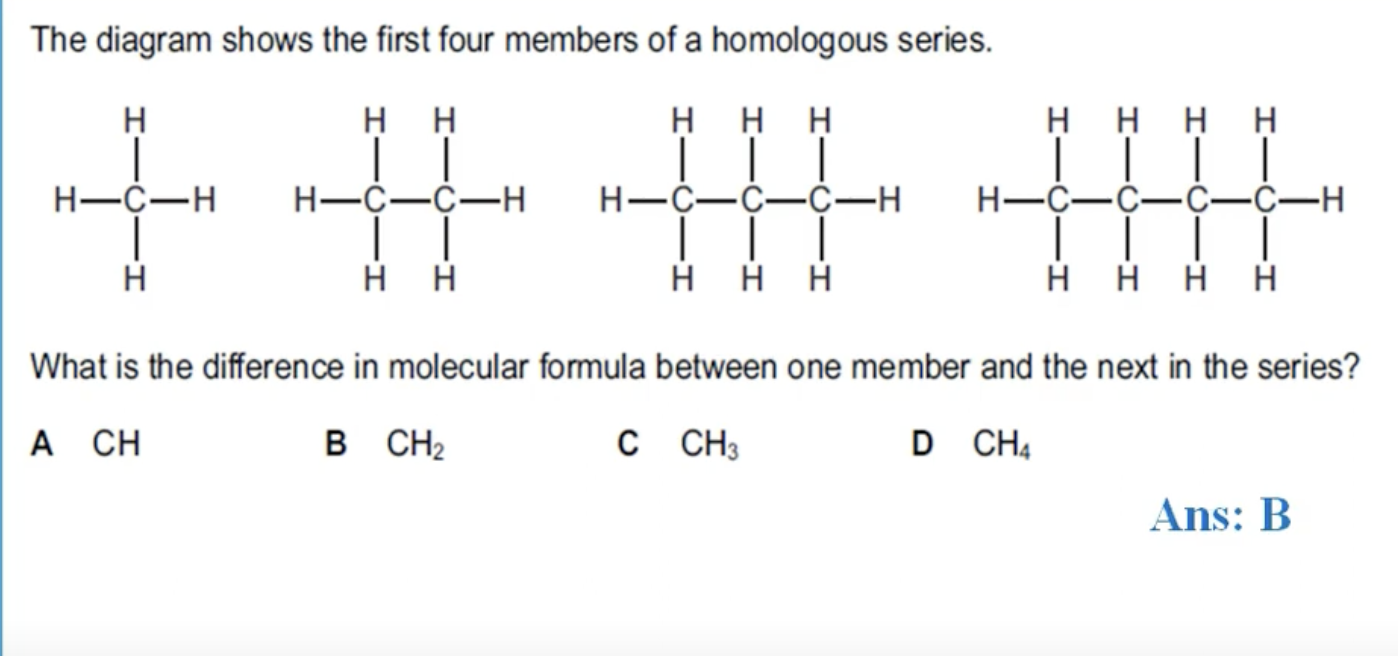
WHAT IS THE ANSWER FOR THIS QUESTION
YOU ARE ADDING ONE CARBON AND TWO HYDROGEN ATOMS.
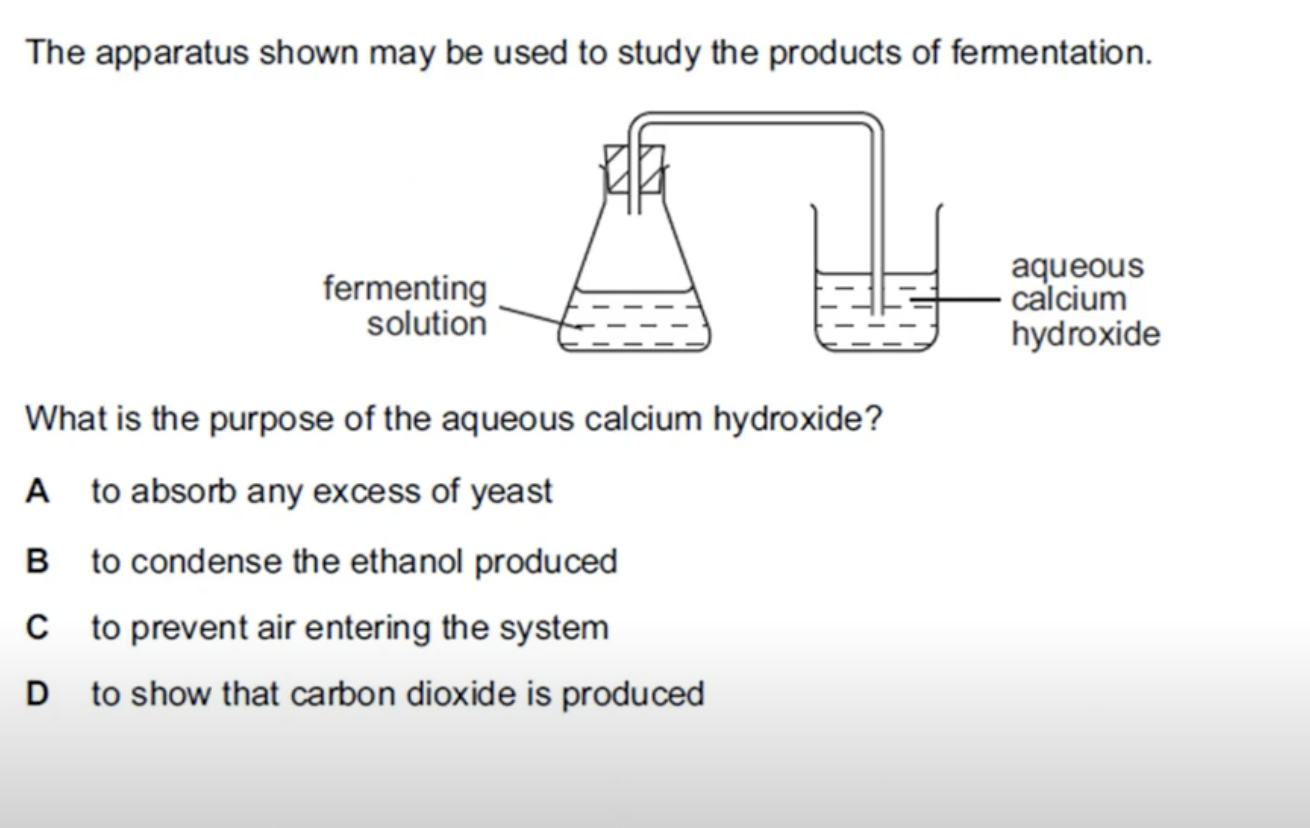
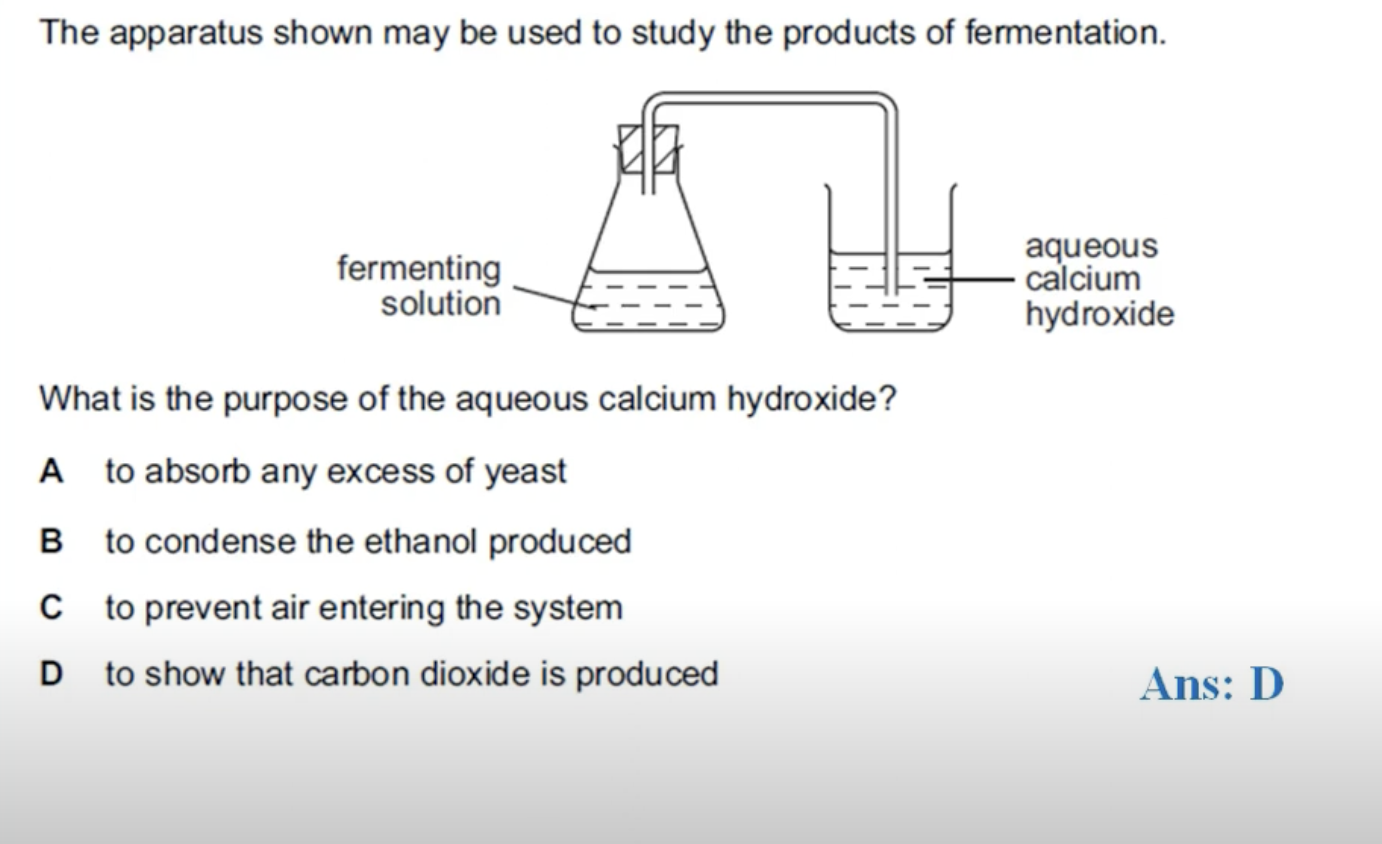
WHAT IS THE ANSWER FOR THIS QUESTION
CALCIUM HYDROXIDE IS LIMEWATER AND LIMEWATER TURNS MILKY IN THE PRESENCE OF CARBON DIOXIDE. IN FERMENTATION PROCESS YOU GET THE ETHANOL AND CARBON DIOXIDE AS PRODUCTS. ETHANOL NEEDS TO BE PURIFIED AS IT IS A MIXTURE OF YEAST, WATER AND ETHANOL.
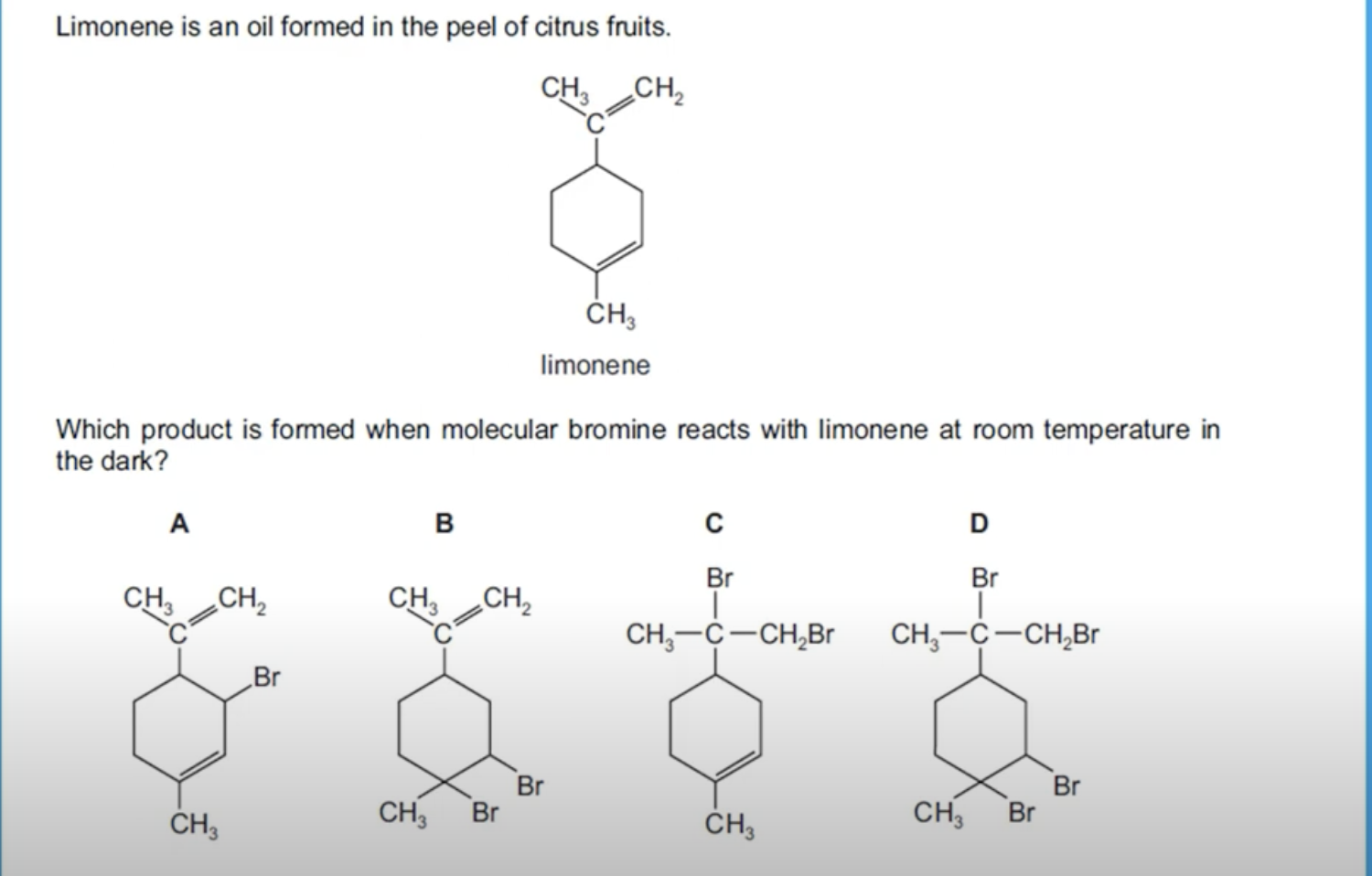
WHAT IS THE ANSWER FOR THIS QUESTION
IT IS C. SINCE, IT IS AN ADDITION REACTION THE DOUBLE BOND WILL BREAK. ANOTHER THING IS THAT IT IS REACTING WITH MOLECULAR BROMINE(Br2) WHICH HAS ONLY TWO BROMINE ATOMS. THIS LEAVES C AS THE ANSWER.

WHAT IS THE ANSWER FOR THIS QUESTION
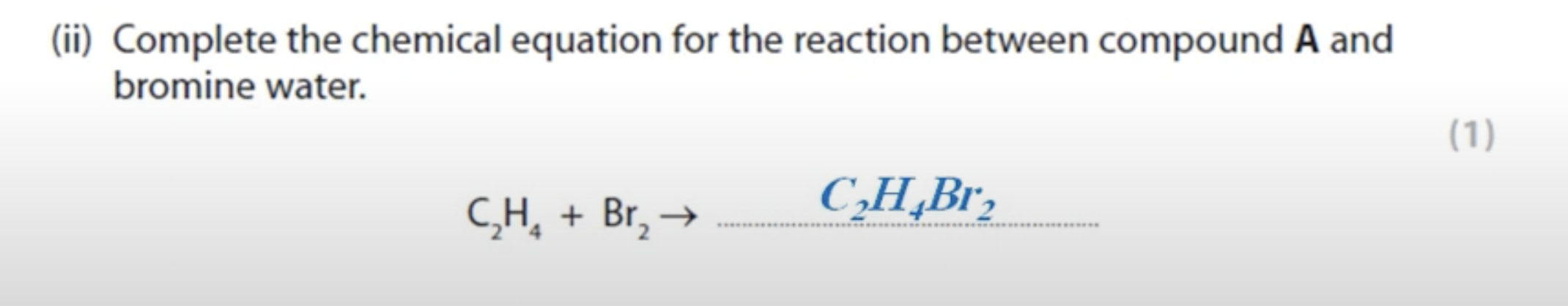

WHAT IS THE ANSWER FOR THIS QUESTION

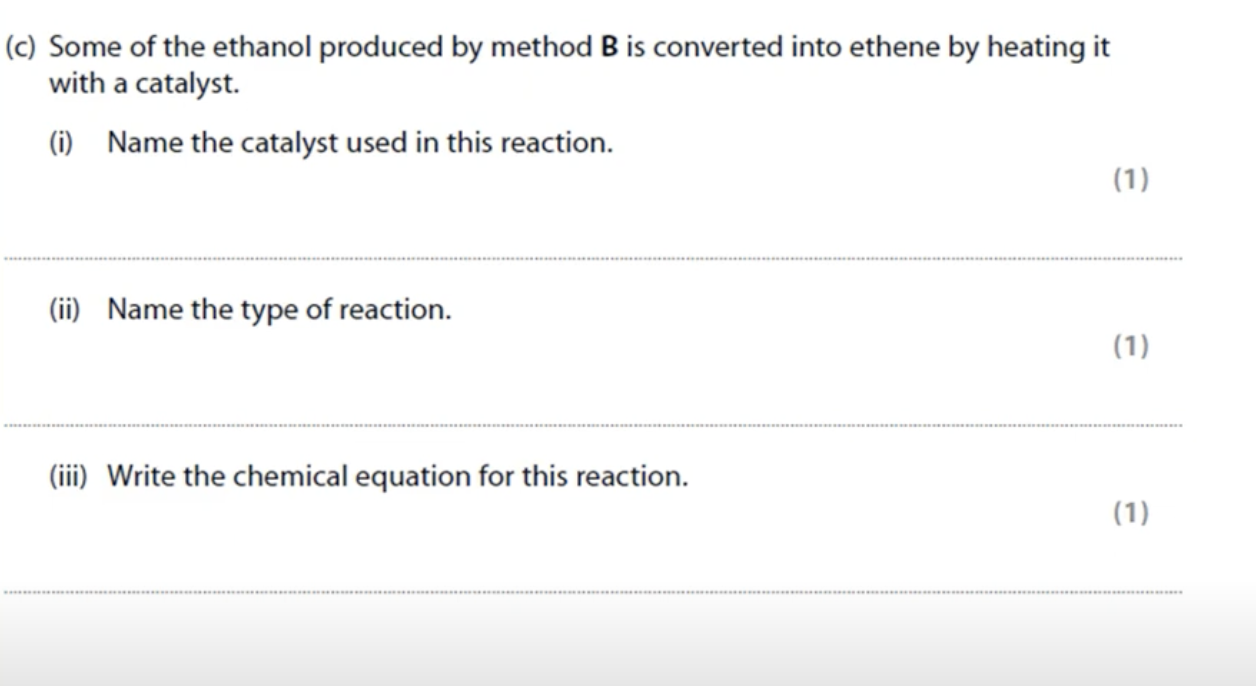
WHAT IS THE ANSWER FOR THIS QUESTION

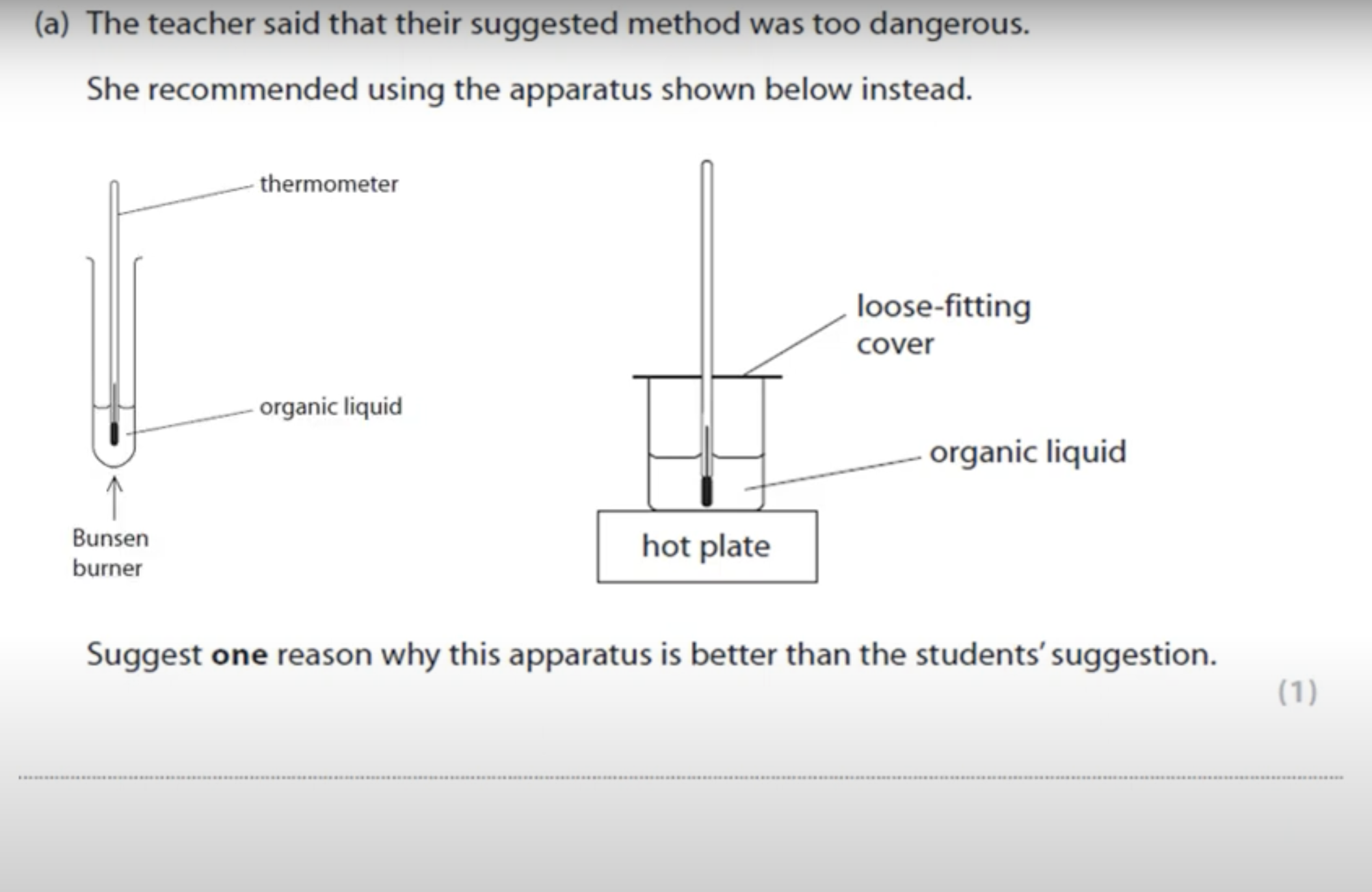
WHAT IS THE ANSWER FOR THIS QUESTION
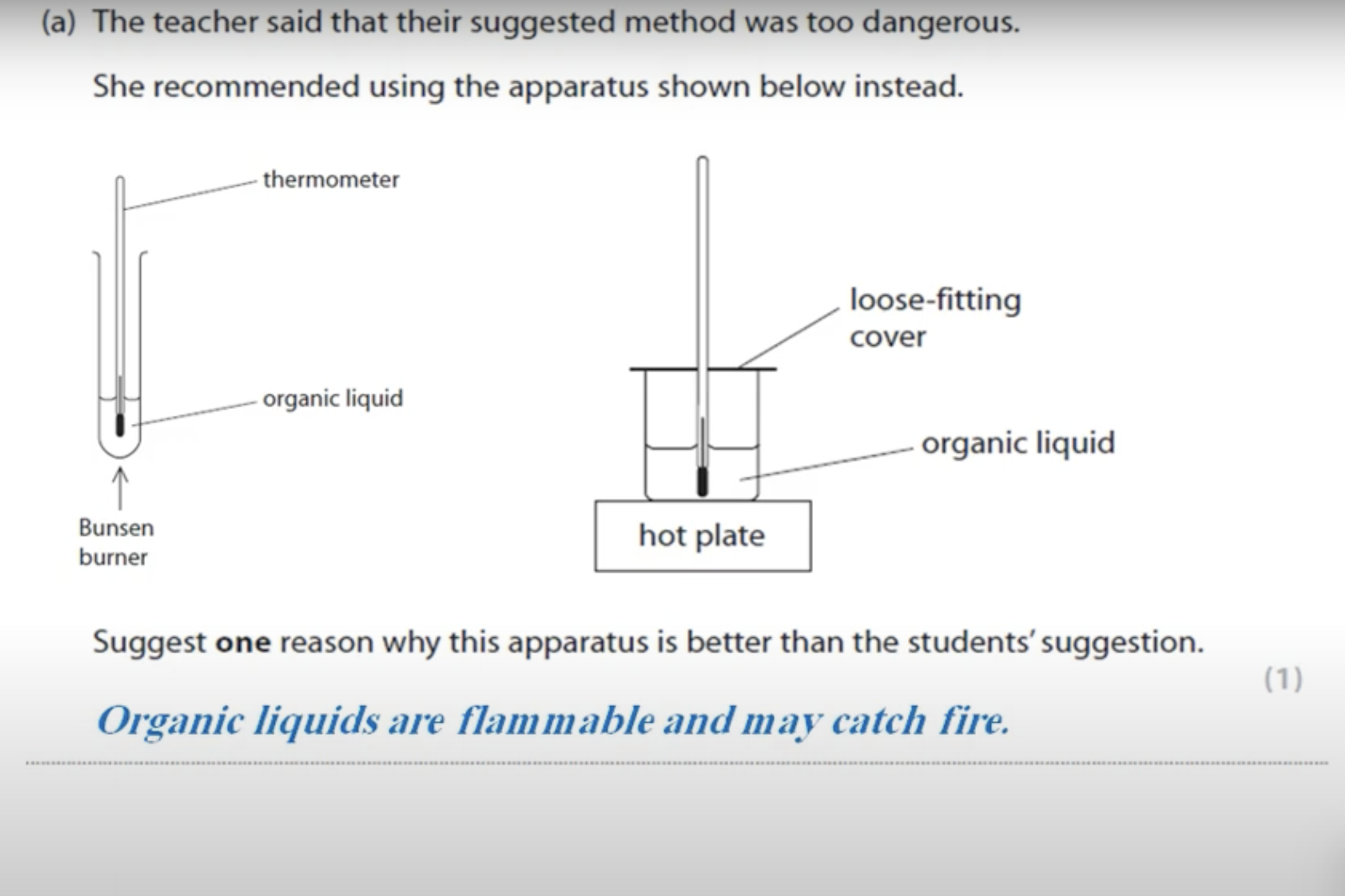
WHAT IS THE ANSWER FOR THIS QUESTION
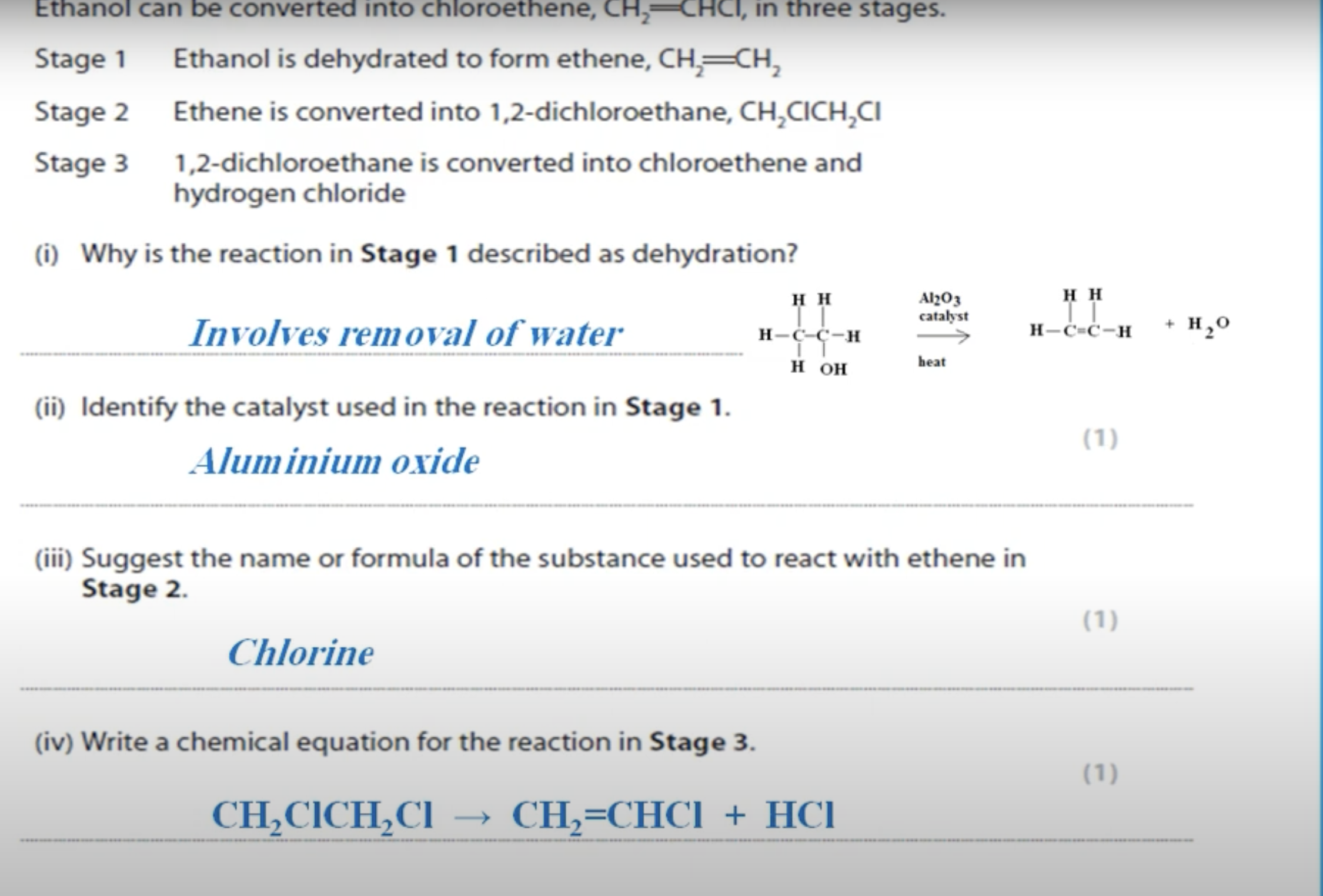

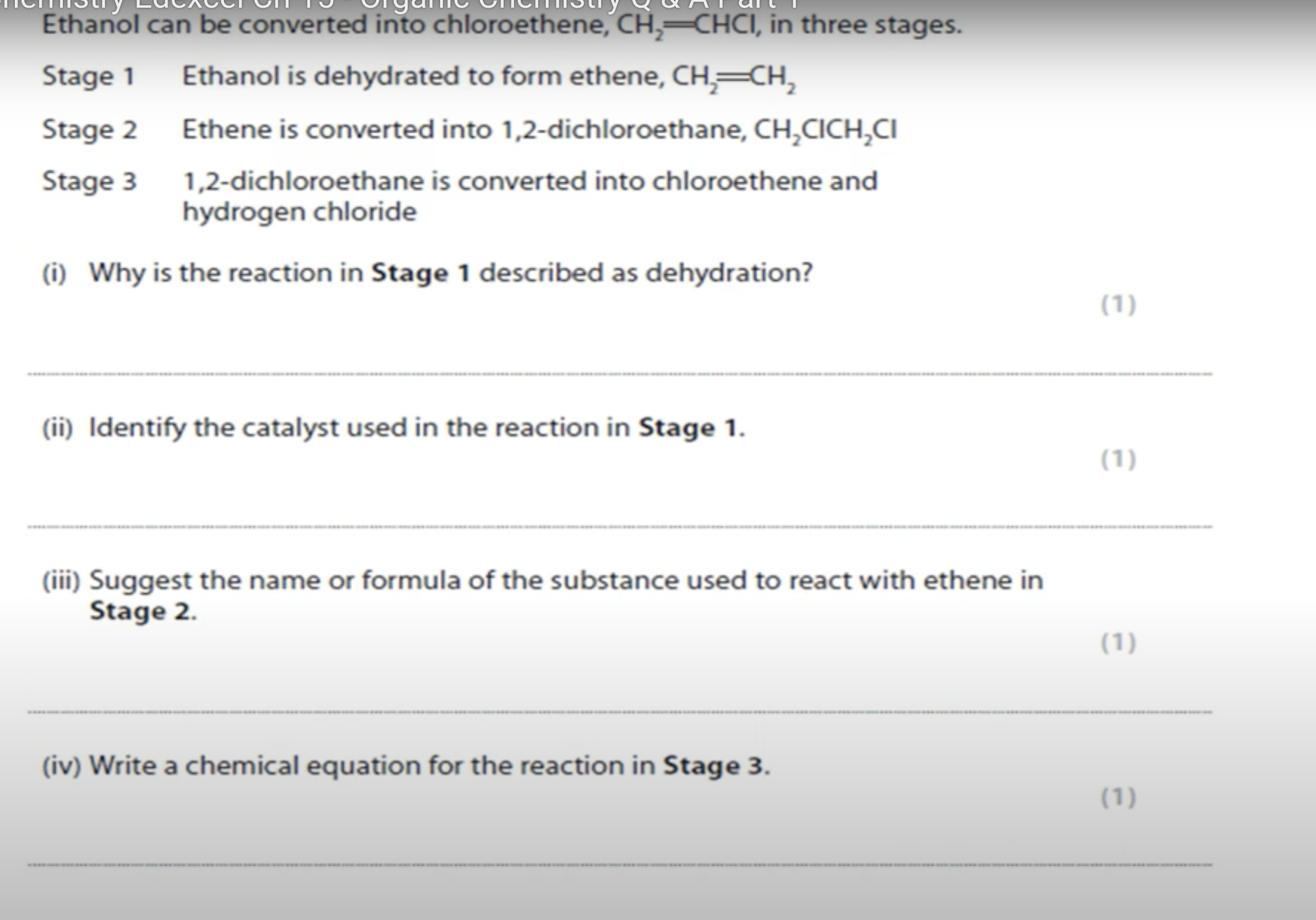
WHAT IS THE ANSWER FOR THIS QUESTION


WHAT IS THE ANSWER FOR THIS QUESTION
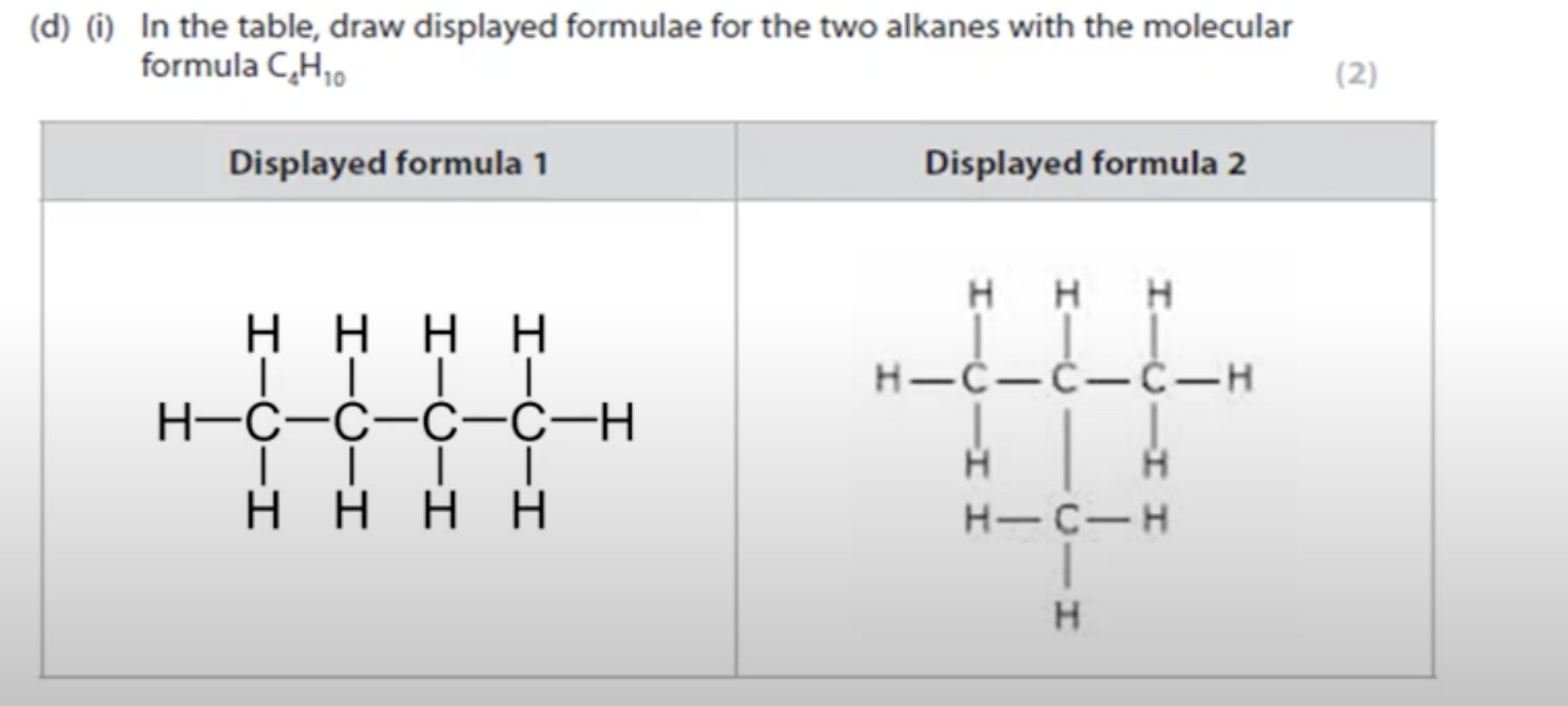

WHAT IS THE ANSWER FOR THIS QUESTION

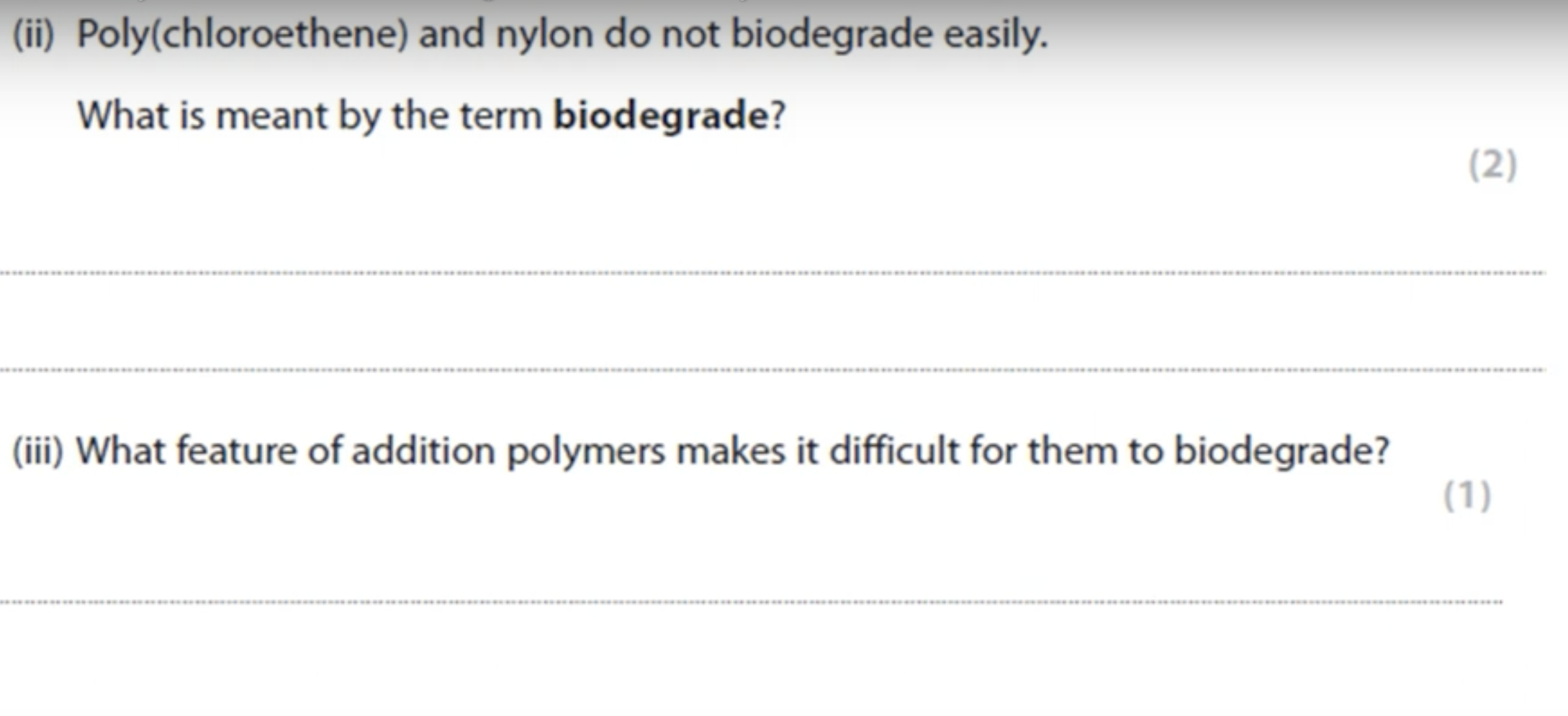
WHAT IS THE ANSWER FOR THIS QUESTION

IN WHICH REACTIONS IS ALUMINIUM OXIDE CATALYST USED
CRACKING
CONVERSION OF ETHANOL TO ETHENE
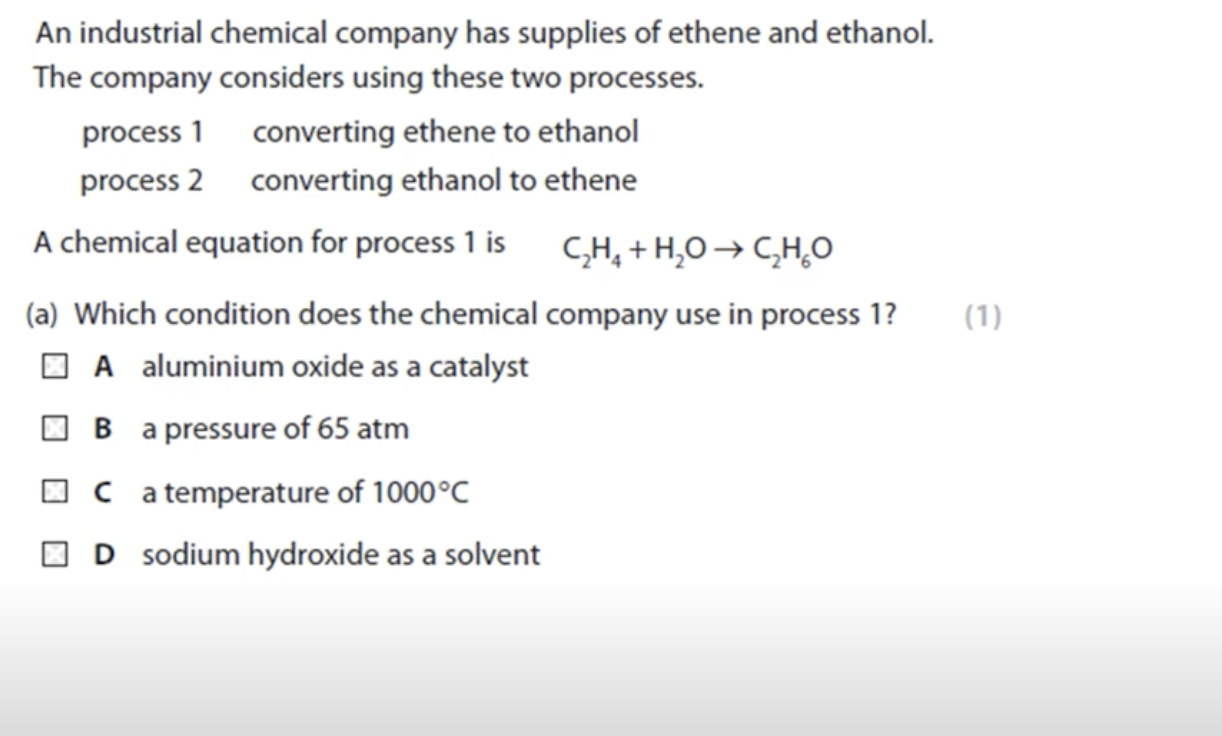
WHAT IS THE ANSWER FOR THIS QUESTION
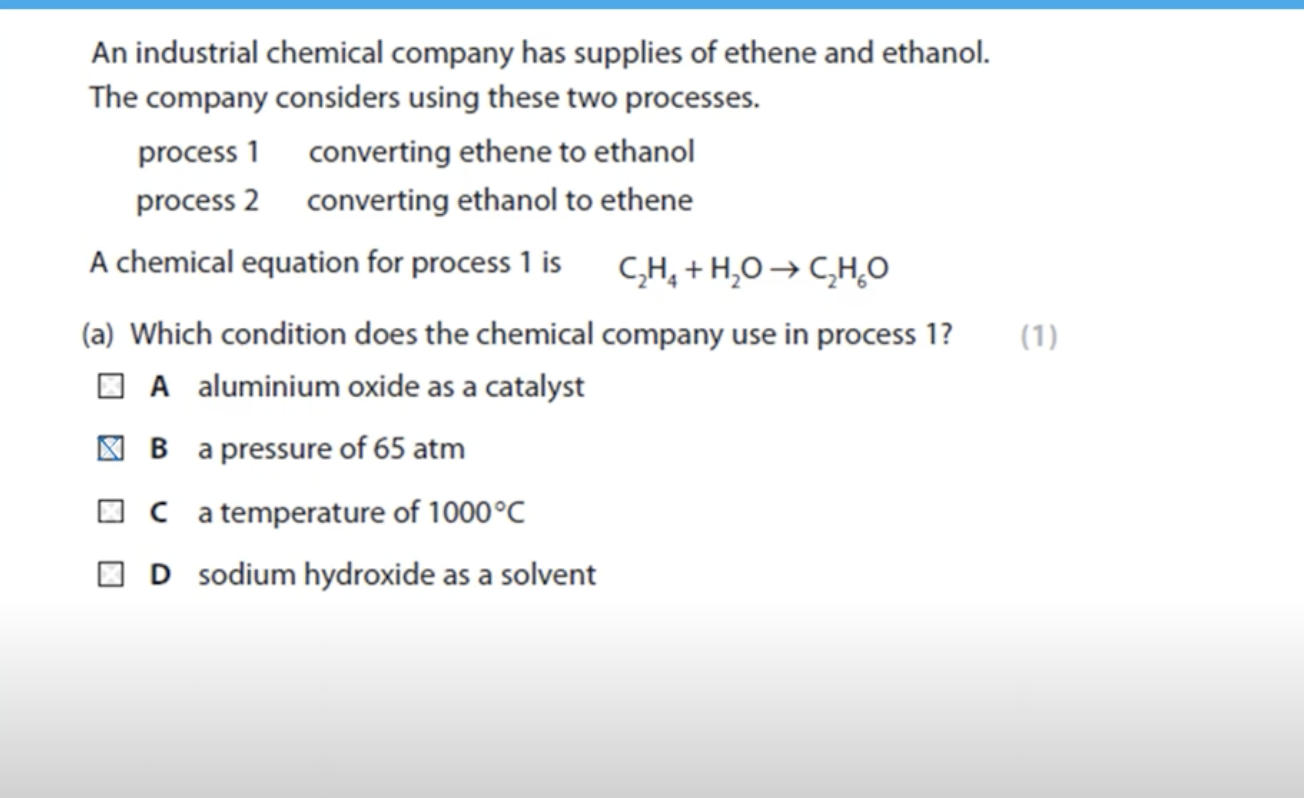

WHAT IS THE ANSWER FOR THIS QUESTION
PROCESS 1 WILL NOT BE EASILY ACCESSIBLE BECAUSE CRUDE OIL IS USED TO GET ETHENE THROUGH FRACTIONAL DISTILLATION
PROCESS 2 WILL BE MORE PREFERRED AS GROWING SUGAR CANE AND IT IS USED TO OBTAIN ETHANOL THROUGH FERMENTATION.
CAN YOU TELL THE COLOUR CHANGE OF METHYL ACID IN:
ACIDIC
NEUTRAL
ALKALINE
ACIDIC- RED-ORANGE
NEUTRAL- YELLOW
ALKALINE- YELLOW
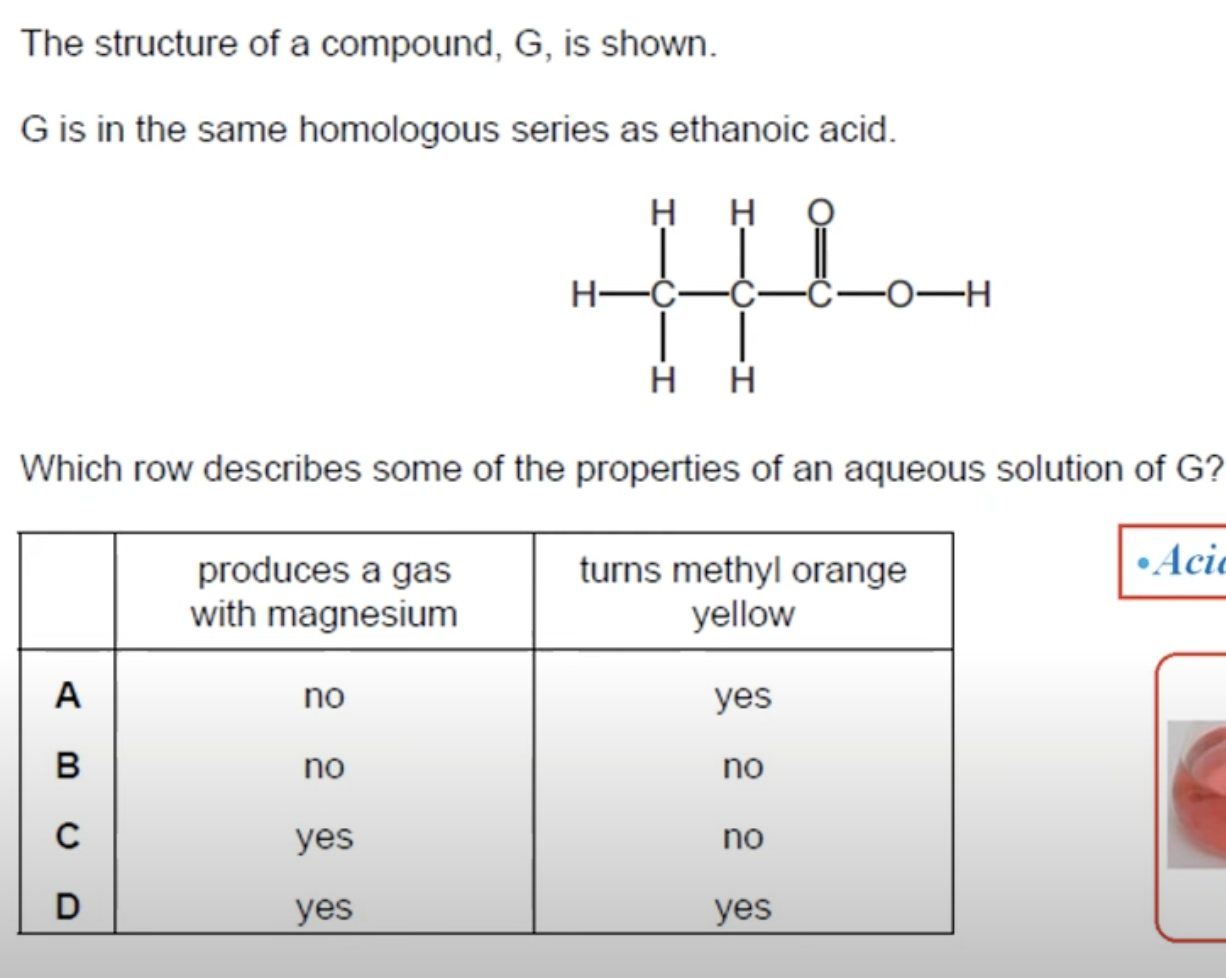
WHAT IS THE ANSWER FOR THIS QUESTION
CARBOXYLIC ACIDS ARE WEAK ACIDS.
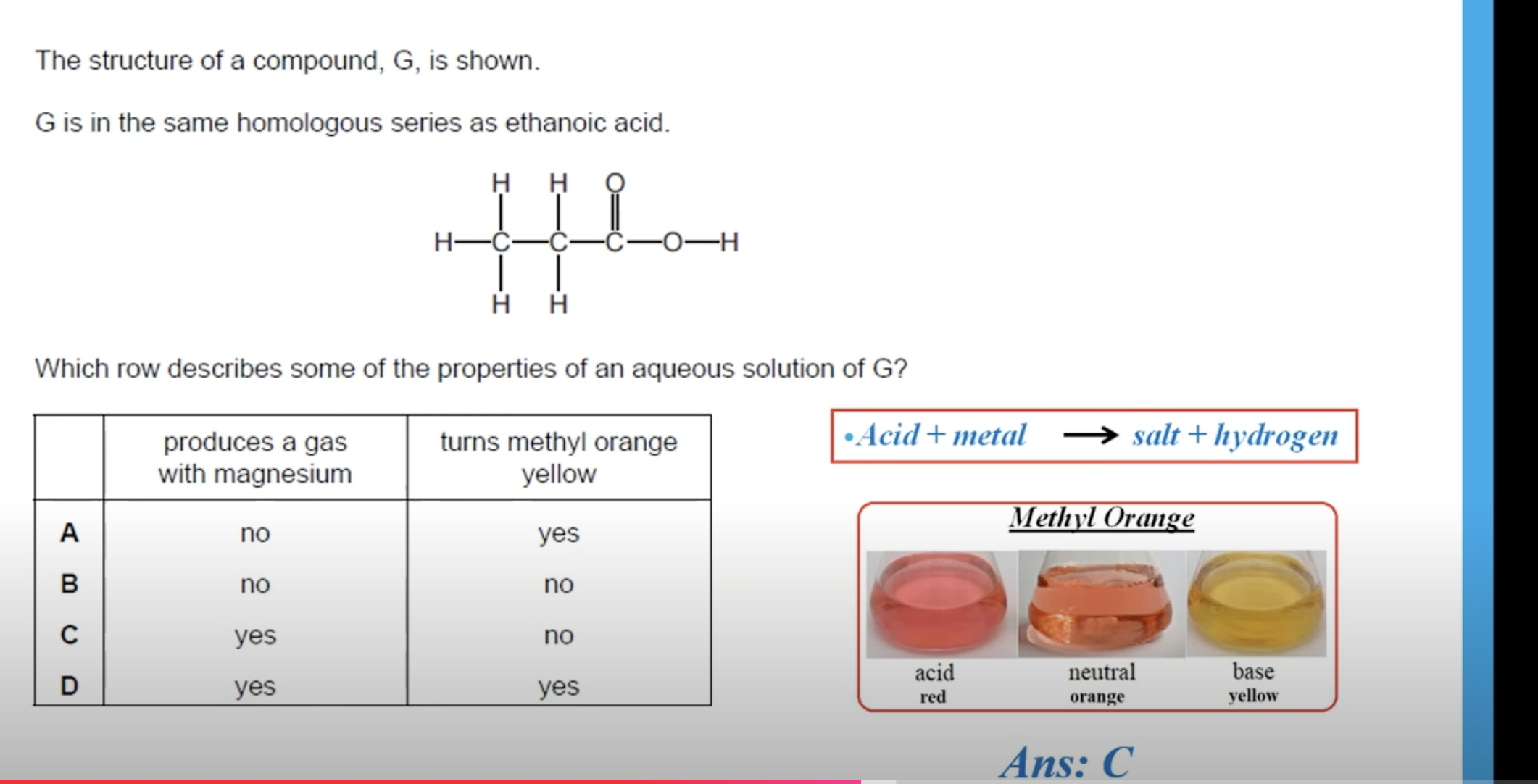
WHAT IS THE ANSWER FOR THIS QUESTION
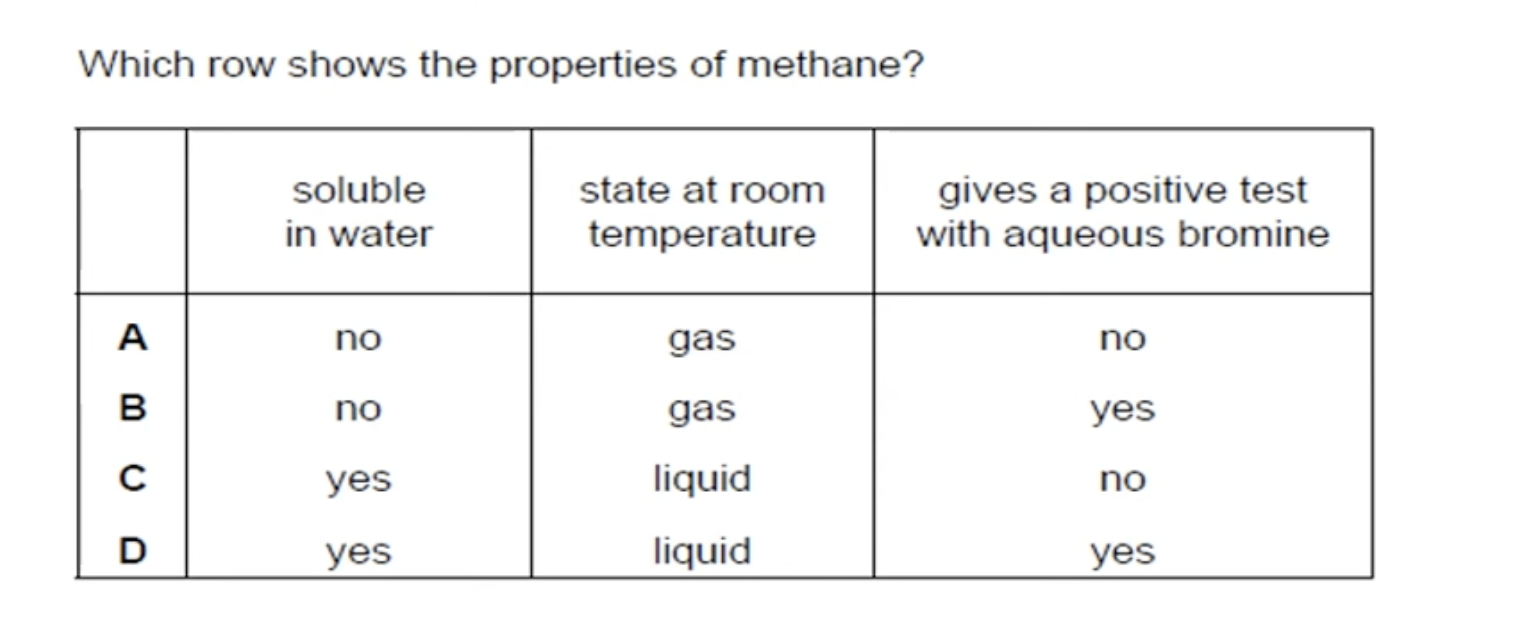
HYDROCARBONS ARE NOT USUALLY SOLUBLE IN WATER.
HYDROCARBONS WITH 5 ATOMS AND ABOVE ARE IN LIQUID STATE, BELOW THAT THEY ARE IN GASEOUS STATE.
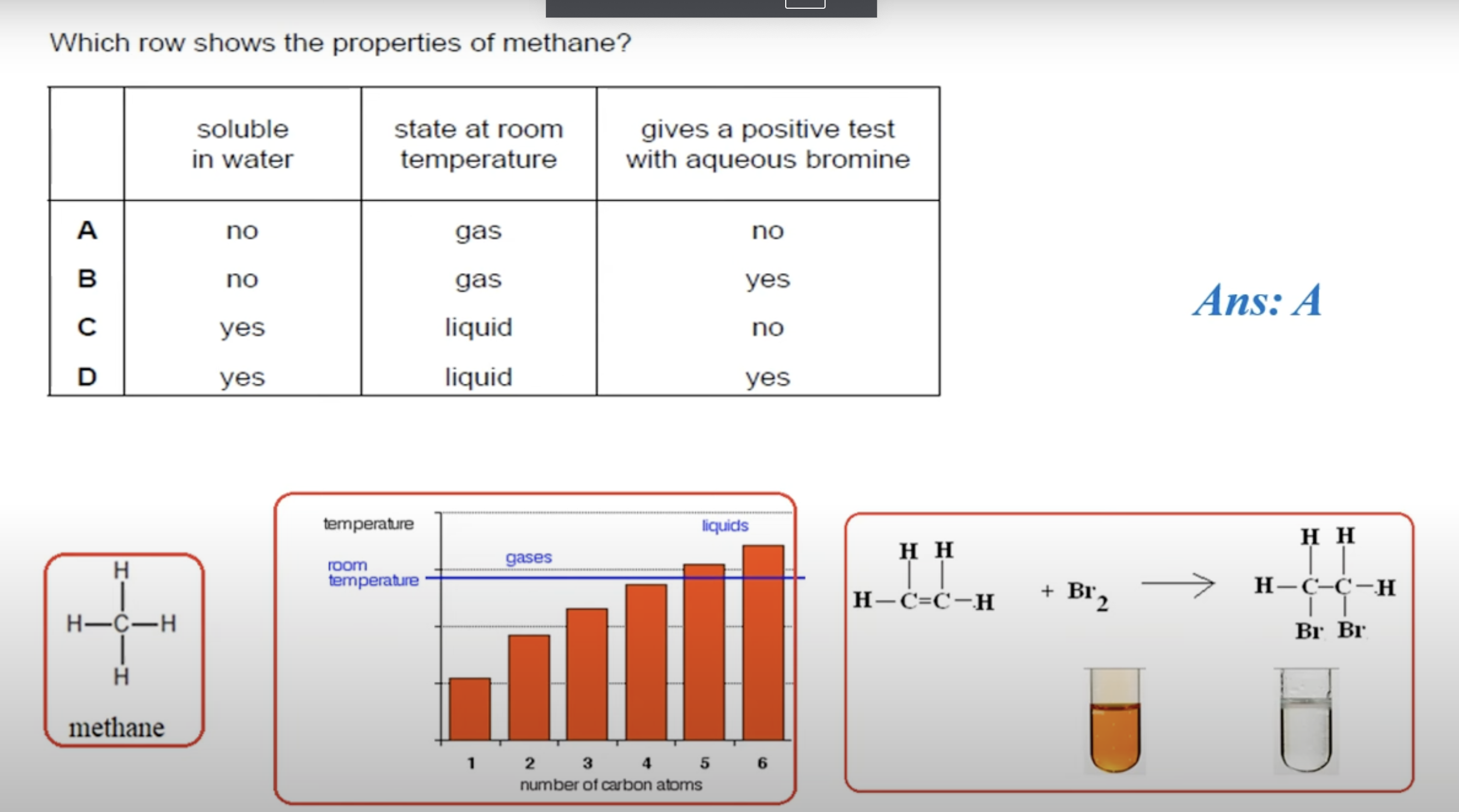
WHAT IS THE ANSWER FOR THIS QUESTION
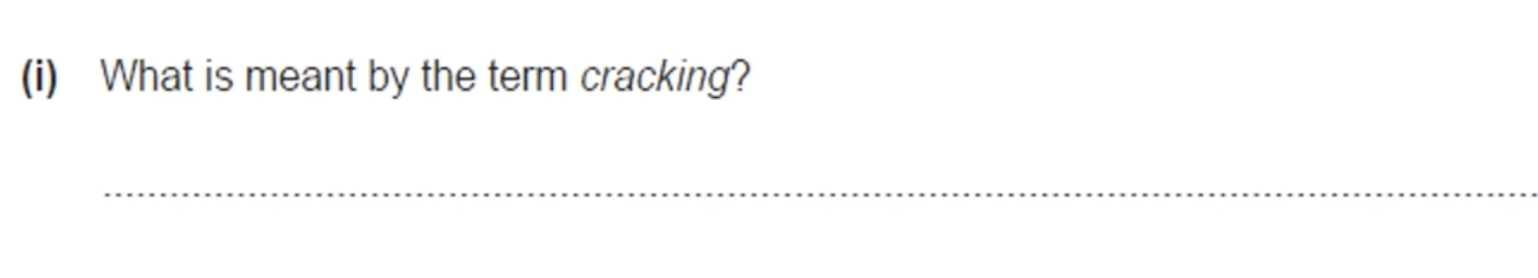
BREAKDOWN OF LONG CHAIN HYDROCARBONS INTO SHORTER CHAIN HYDROCARBONS USING HEAT AND A CATALYST.
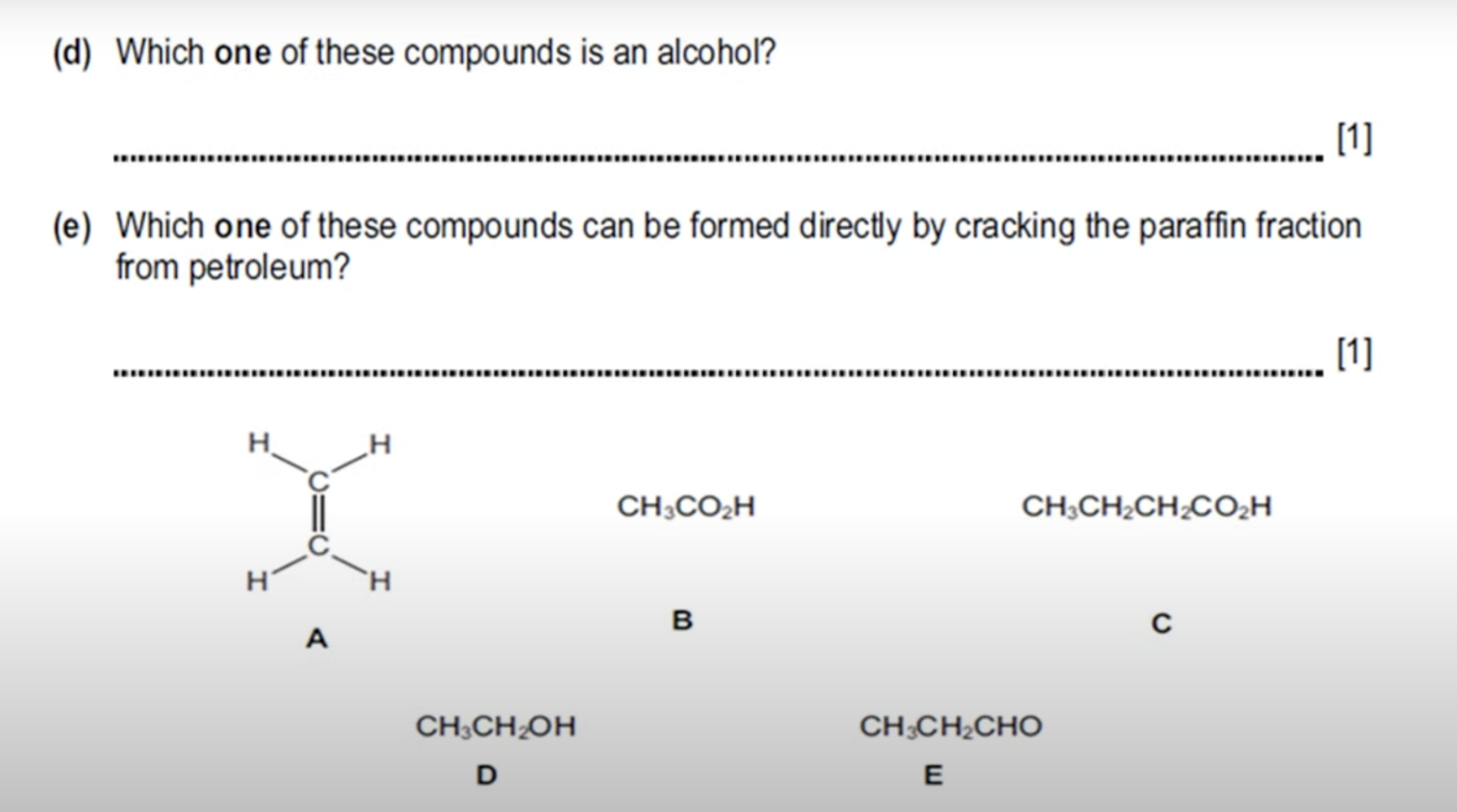
WHAT IS THE ANSWER FOR THIS QUESTION
CH₃CH₂CHO: THIS IS NOT AN ALCOHOL BUT AN ALDEHYDE CONSISTING OF A CARBONYL CARBON(C=O) BONDED TO A HYDROGEN ATOM—→ [NOT IN SYLLABUS]
FOR IT TO BE AN ALCOHOL IT IS SUPPOSED TO BE (-OH) GROUP.
B AND C ARE BOTH CARBOXYLIC ACIDS AS THEY HAVE THE GROUP CO2H(THE O2 MEANS TWO OXYGEN ATOMS, THUS MAKING IT COOH WHICH IS THE FUNCTIONAL GROUP OF CARBOXYLIC ACIDS).
![<p>CH₃CH₂CHO: THIS IS NOT AN ALCOHOL BUT AN ALDEHYDE CONSISTING OF A CARBONYL CARBON(C=O) BONDED TO A HYDROGEN ATOM—→ [NOT IN SYLLABUS]</p><p>FOR IT TO BE AN ALCOHOL IT IS SUPPOSED TO BE (-OH) GROUP. </p><p> B AND C ARE BOTH CARBOXYLIC ACIDS AS THEY HAVE THE GROUP CO<sub>2</sub>H(THE O<sub>2 </sub>MEANS TWO OXYGEN ATOMS, THUS MAKING IT COOH WHICH IS THE FUNCTIONAL GROUP OF CARBOXYLIC ACIDS). </p>](https://knowt-user-attachments.s3.amazonaws.com/a4ac2c9e-e75a-40db-ba20-5d40438fd618.png)

What is the answer for this question?
Sulphuric acid

What is the answer for this question?
In an alkene you need AT LEAST 2 carbon atoms for there to be a double bond, thus the first member of alkenes is going to be ethene and likewise the second member is propene.
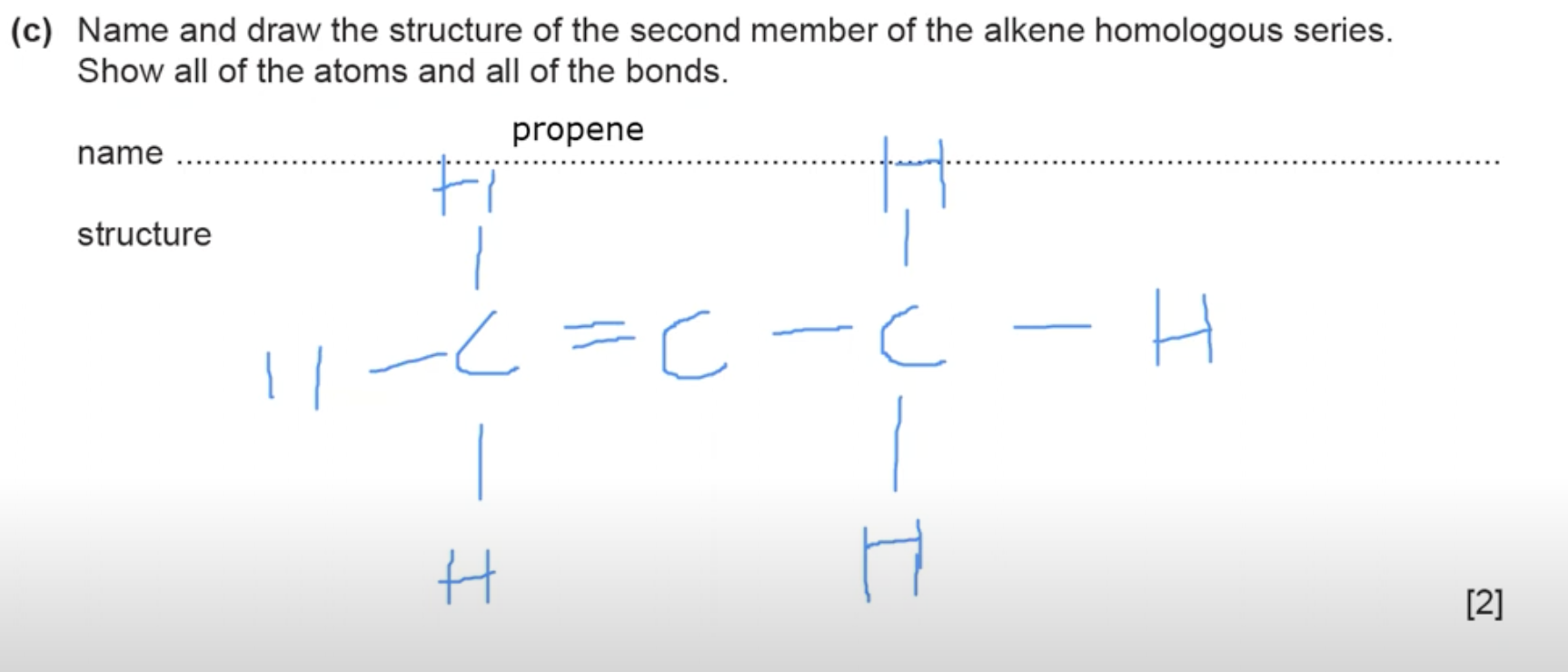

What is the answer for this question?
ACIDIFIED POTASSIUM MANGANATE(VII)

What is the answer for this question?
More than enough oxygen present to react
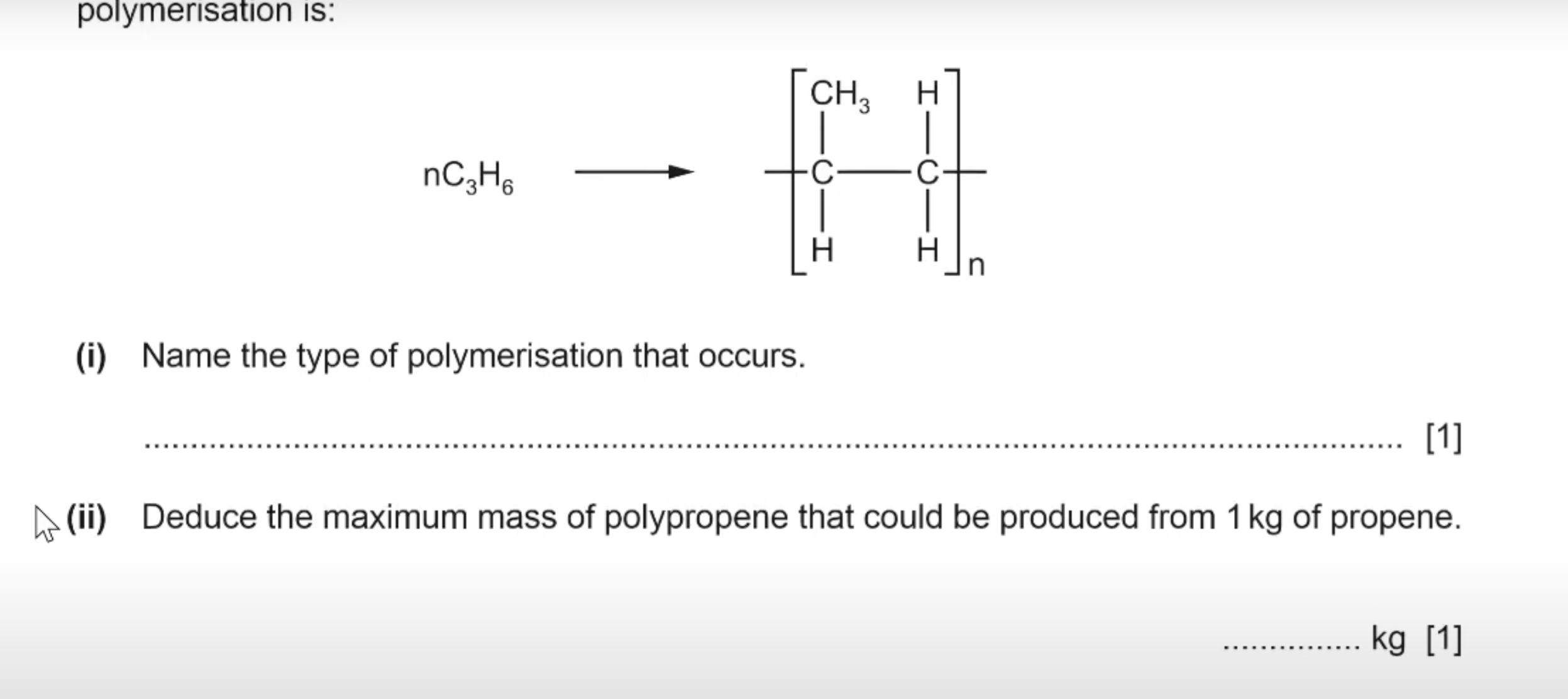
What is the answer for this question?
Addition polymerisation
1 kg of polypropene. This is because in addition reaction you only get the polymer as the product, thus one kg of the reactant(propene) will give one kg of the product(polypropene).