exam 4: enzyme mechanisms and regulation
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
what are the 4 mechanisms of catalysis
covalent catalysis
acid-base catalysis
low-barrier hydrogen bonds
metal ion catalysis
what are the biochemical nucleophiles
serine (-OH)
cysteine (-SH)
lysine (-NH2)
histidine (imidazole group)
water (H2O)
what is a nucleophile
an electron-rich species that donates a pair of elections to an electrophile to form a chemical bond
chymotrypsin, a protease enzyme uses __
both covalent and acid/base catalysis
what is covalent catalysis
the enzyme forms a termporary covalent bond with the substrate, creating a more reactive intermediate that facilitates the reaction
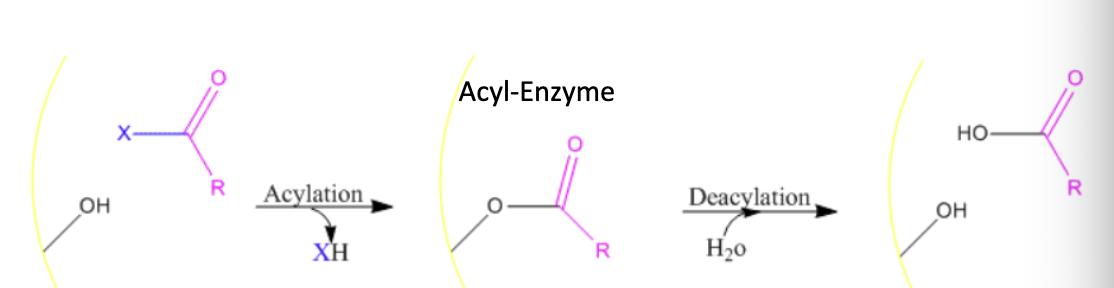
what does chymotrypsin cleave
the carboxyl side of large hydrophobic or aromatic amino acids such as phe, met, tyr, and trp
what are the mechanistic steps of chymotrypsin
Substrate binds to enzyme
nucleophilic residues in active site attack an electrophilic center on S, creating a covalent bond
tetrahedeal intermediate is formed, the enzyme stabilizes the high energy transition state
product formed through rearrangement or bond cleavage
water attacks the intermediate to break the EP complex
product is released
what is the overall process of acid-base catalysis
a proton is transferred to catalyze reactions
what are the mechanistic steps of acid-base catalysis
nucleophilic attack of base, proton transfer, product forms
what is specific acid-base catalysis involve
H+ or OH- that diffuses into the catalytic center (strong acid or strong base)
what does the rate of a specific acid-base reaction depend on
the pH of the solution, not concentration of the buffer
what does general acid-base catalysis involve
acids and bases other than H+ and OH-
what does the rate of a general acid-base reaction depend on
depends on both the pH and the concentration of the buffer
what are examples of enzymes that employ general acid-base catalysis
serine and aspartic acid
what is a low-barrier bydrogen bond (LBHB)
special type of H bond where the proton is more equally shared between the donor and acceptor atoms, rather than being strongly associated with one
exceptionally strong and play a critical role in enzyme catalysis by stabilizing transition states and lowering activation energy
strength: 10-30 kj/mol
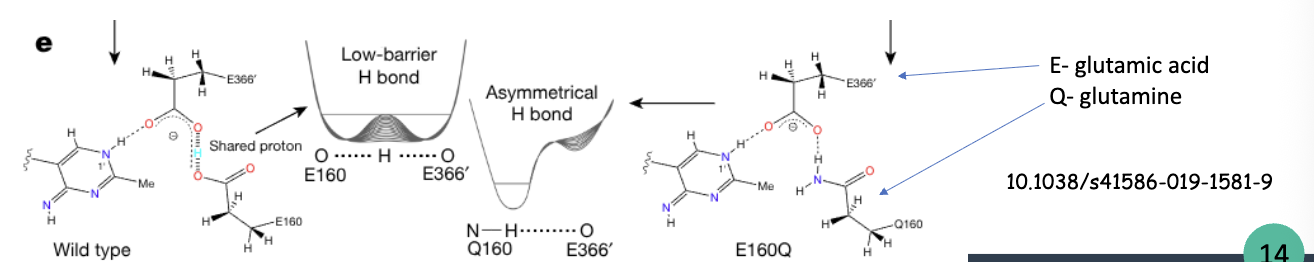
what are the mechanistic steps of LBHBs
S binds to E via H bonding: Asp, Glu, His, Tyr are common residues for H bonding
hydrogen bond strengthening, the H bond is shortened. transition state is stabilized
transition state collapses through bond cleavage or formation
LBHB reverts to normal H bond
what is metal ion catalysis
metal ions in an enzymes cofactors interact with the substrate, stabilizing the transition state and orienting the substrate for reaction
what are the mechanistic steps of metal ion catalysis
water activates
substrate binds
leaving group leaves
what are the 3 types of enzyme regulation
the presence of allosteric regulators or inhibitors
genetic regulation
compartmentation
what enzyme converts ATP into cAMP
Adenylyl cyclase
what is cAMP
the intracellular agent of extracellular hormones: a second messenger used in intracellular signaling (PKA activation)
how is cAMP formed from ATP
the 3’ hydroxyl on ribose attacks the alpha-phosphate of ATP, forming a 3’-5’ cyclic phosphate ring
why does breaking down pyrophosphate matter
hydrolysis of PPi is highly exergonic which drives the formation of cAMP forward and makes the reaction effectively irreversible
what does the binding of Ga(GTP) to adenylyl cyclase do
activate cAMP production
what is cAMP-dependent protein kinase composed of
catalytic and regulatory subunits
what do the two regulatory subunits bind in cAMP-dependent protein kinase
two equivalents of cAMP each
cAMP binding releases the R subunits from the catalytic subunits
which amino acid side chain is involved with adenylation
tyrosine
which amino acid side chain is involved with uridylylation
tyrosine
which amino acid side chain is involved with ADP-ribosylation
arginine
which amino acid side chain is involved with redox
cysteine
which amino acid side chain is involved with acetylation
lysine
what is transcriptional control
gene expression can be upregulated or downregulated in response to cellular needs.
ex: lac operon in proks

what does the presence of lactose upregulate
the expression of lacZ, lacY, and lacA genes
why are enzymes, substrates, and regulatory molecules located in separate compartments/areas
so that opposing pathways are physicallyseparated, or located close together, to incraese pathway efficiency