Biology A level term1
1/236
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
237 Terms
What is a monomer?
Single subunit in a polymer
What is a polymer?
Many monomers joined together
What is a condensation reaction?
When a bond is formed between two molecules, resulting in the removal of water
What is a hydrolysis reaction?
Breaking of a bond between two monomers by the addition of a water molecule
What are monosaccharides?
Monomers from which larger carbohydrates are made
How do disaccharides form?
From the condensation of 2 monosaccharides
How do polysaccharides form?
By the condensation of many monosaccharide units
What is a glycosidic bond?
Bond formed from a condensation reaction between 2 monosaccharides
What is an isomer?
Compound with same molecular formula (number and type of atoms) but different arrangements of those atoms
What is starch?
A polysaccharide made of alpha glucose monomers that is used as the main storage of glucose in plants
What is glycogen?
Highly branched polysaccharide made of alpha glucose monomers that is used as the main storage of glucose in animals
What is cellulose?
Polysaccharide made of beta glucose monomers that is used as a structural polysaccharide which provides strength to plant cell walls
What do nucleotides turn into during condensation reaction?
Polynucleotides (DNA and RNA)
What do monosaccharides turn into during condensation reaction?
Polysaccharides (starch)
What do amino acids turn into during condensation reaction?
Polypeptides (Proteins)
What are the uses of carbohydrates?
Stores glucose and can provide structural support to plant cells
Monosaccharides are…
Soluble and sweet e.g. glucose, fructose and galactose
Glucose is a hexose sugar, meaning…
It has six carbon atoms, resulting in the formula C6H12O6
Isomers of glucose can occur, which are…
α-glucose
β-glucose
What is the structure α-glucose?
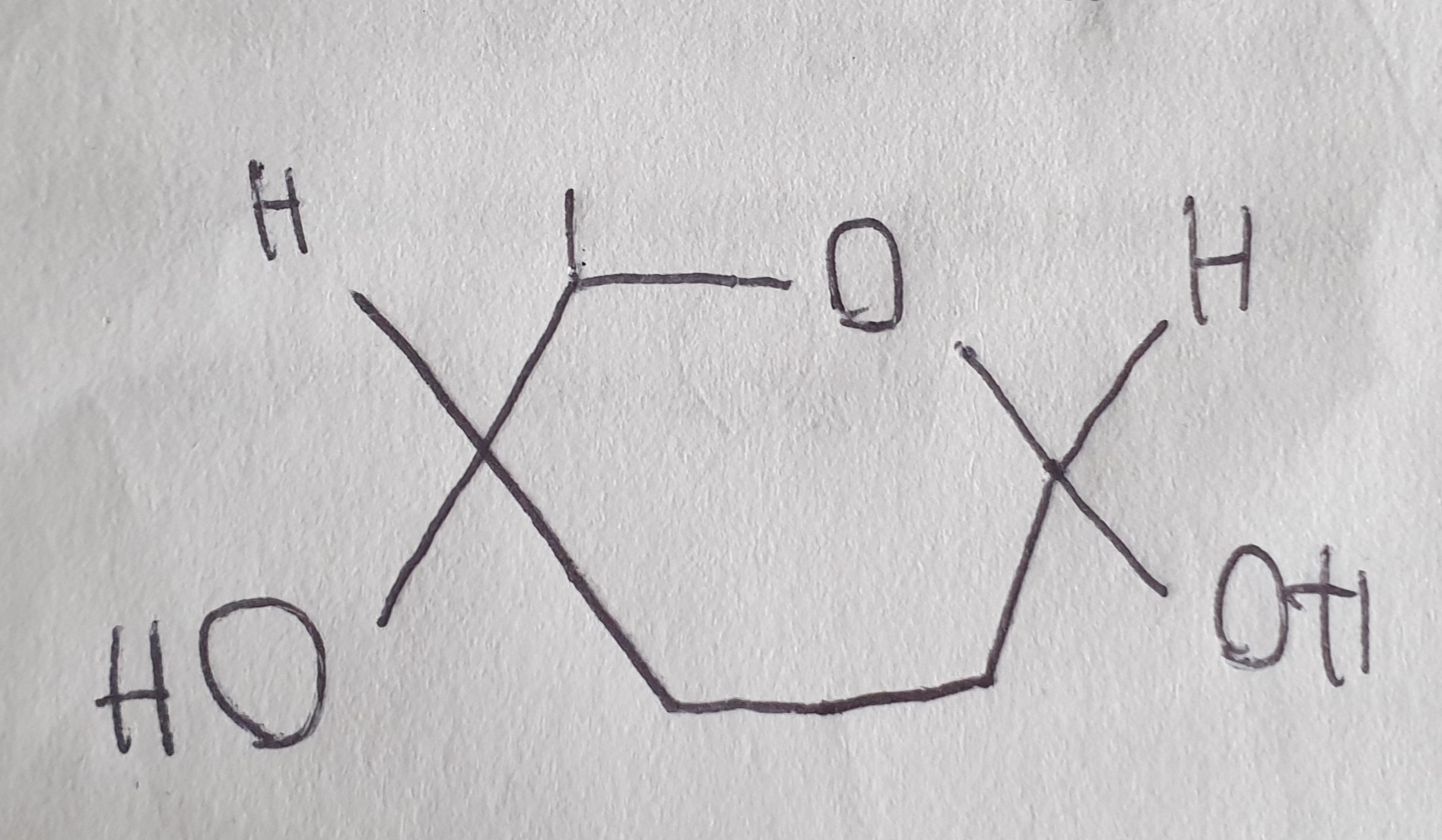
What is the structure of β-glucose?
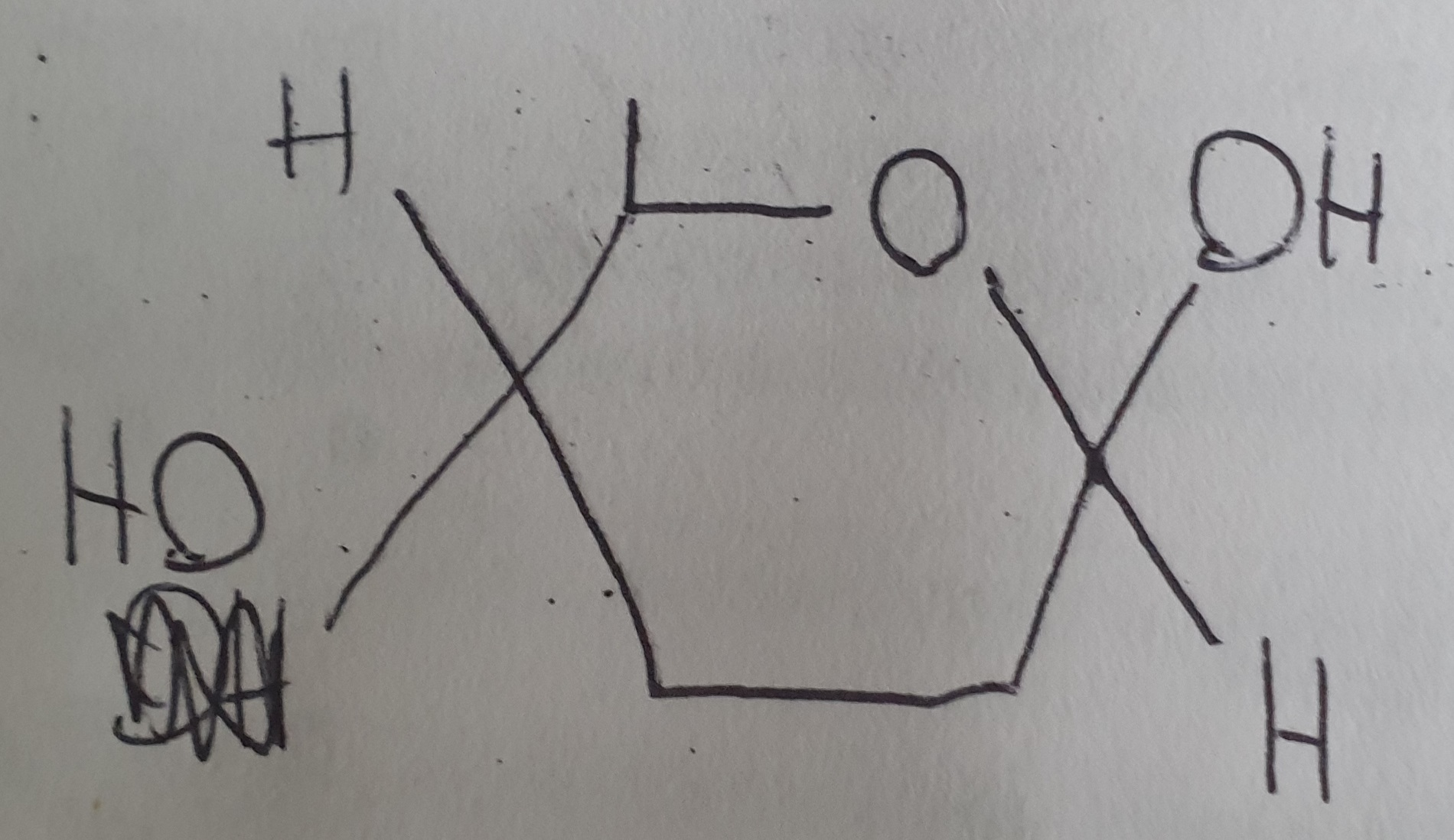
What is the difference between α and β glucose?
Both are isomers of each other other, they have a different structure
α glucose forms glycogen and starch
β glucose forms cellulose
Glucose + Galactose =
Glucose + Fructose =
Glucose + Glucose =
Lactose
Sucrose
Maltose
Pick the image of the formation of maltose
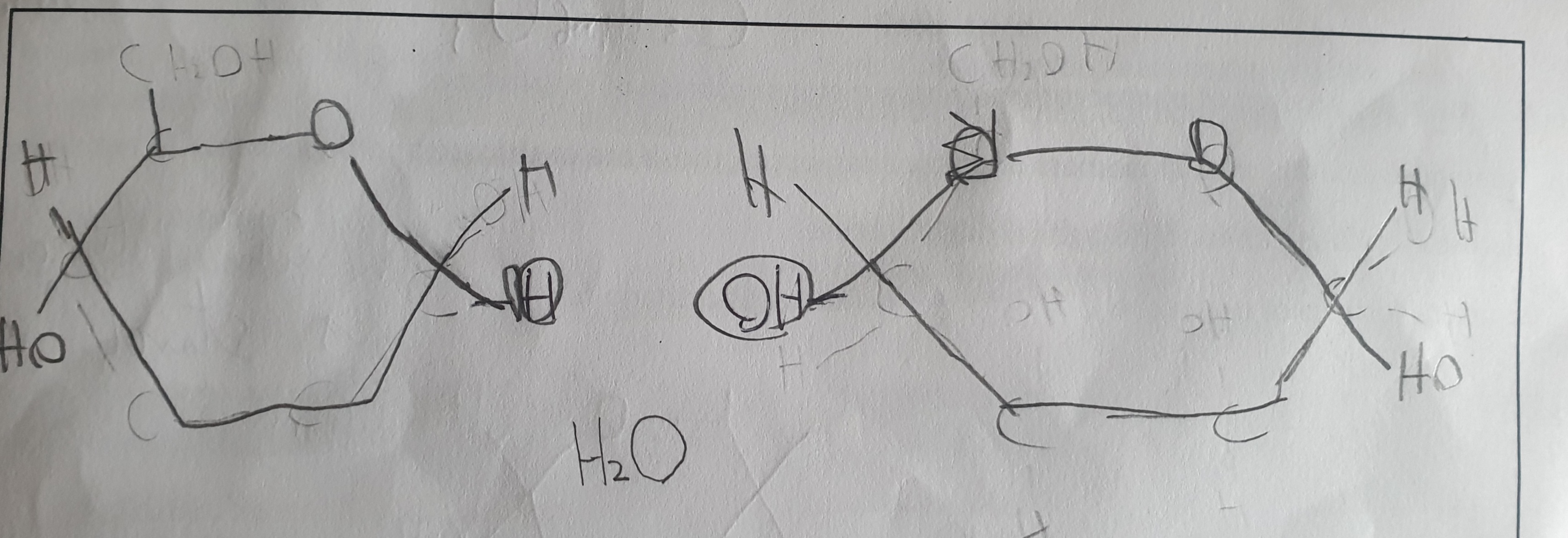
Give the symbol equation for the synthesis of maltose, sucrose and lactose
C6H12O6 + C6H12O6 → C12H22O11 + H2O
What are the 3 important polysaccharides?
Starch - chains of α-glucose (glucose storage in plants)
Cellulose - chains of α-glucose (glucose storage in animals)
Glycogen - chains of β-glucose (structural support in plant cell walls)
What are the 2 different polymers that starch is made off and give details about them?
Amylose forms a compact helical chain to store large numbers of glucose molecules in a small space
Amylopectin is branched, providing a large surface area for enzymes to attach, meaning starch is readily hydrolysed back into glucose when plant cells are running low on glucose for respiration
What is glycogen when compared to starch?
Found in animals and is similar to amylopectin, but has more branches. It’s an energy storage molecule in animals and is found in liver and muscle cells
Glycogen and starch are well suited as glucose storage molecules because…
They are compact, so stores lots of glucose in a small space
Large and insoluble, so cannot diffuse out of the cells in which they are stored
Insoluble, so no osmotic effects
Relatively inert (chemically inactive), so doesn’t become involved in chemical reactions in the cell
Glycogen (and amylopectin) are quickly hydrolysed to soluble glucose because they have many ends for amylase enzymes to hydrolyse
If you are trying to connect 2 β-glucose, what should you do?
flip the one on the left
Cellulose is a major component of plant cell walls and is suited to this function because…
It’s made up of chains of β-glucose molecules which form straight unbranched chains, so can’t be easily broken down
Chains run parallel to each other and hydrogen bonds form cross-linkages between chains. Many hydrogen bonds are collectively strong and so provide a high tensile strength, making cell wall rigid and prevents osmotic lysis
Cellulose molecules are grouped to form microfibrils, which in turn are grouped to form fibres, providing more strength
What are the shapes of starch, glycogen and cellulose?
Starch - compact helical coil shape (amylose), branched (amylopectin). Allows efficient storage and maintains osmotic balance
Glycogen - Similar to amylopectin but more branched. Compact, highly branched spherical structure, allowing rapid release of glucose.
Cellulose - Straight unbranched chains
How do you do the test for starch?
Add iodine solution to the sample and if the colour changes from orange-brown to a blue-black, starch is present
How do you do the benedict’s test for reducing sugar?
Benedict’s reagent (blue) is added to test solution
Heat for 5 minutes
Sample should go red if reducing sugar is present
What is a reducing sugar?
Those with the ability to donate electrons. All monosaccharides and some disaccharides (like sucrose) are reducing sugars
How do you do the test for non-reducing sugars?
Heat the sample with benedicts’s reagent, if there is no colour change, reducing sugar is not present (but non-reducing might be)
Boil a fresh sample with hydrochloric acid (this will hydrolyse the non-reducing, if present, into its constituent monosaccharides)
Neutralise with sodium hydrogen carbonate
Re-do the benedict’s test and if solution turns red (or green/yellow/brown), explanation is that non-reducing sugar was present in the sample initially
What are the issues related to the benedict’s test?
Non-specific: a positive result just tells us that a reducing sugar is present, but it doesn’t tell us which one, so biosensors can be used to test for specific sugars
Test is qualitative: colour change is used to determine result, so we can’t obtain a value for the concentration of reducing sugar. However, colour change depends upon the amount of reducing sugar present, so test can also be described as semi-quantitative, allowing an estimate of how much reducing sugar is present
It is subjective: colorimeter can be used to quantify results, serial dilution of a glucose solution can be carried out, producing a set of solutions of known concentrations, the benedict’s test can be peformed on each of these and the colour intensity measured using a colorimeter, calibration curve can be produced from the results, which can then be used to read off the concentration of glucose in an unknown solution
What conclusions can be drawn on the amount of sugar from the benedicts sugar test colour change?
Blue - none
Green - very low
Yellow - low
Brown/Orange - medium
Red - high
Lactulose is a disaccharide formed from one molecule of galactose and one molecule of fructose. Other than both being disaccharides, give similarities and differences between the structures of lactulose and lactose
Similarities: Both contain galactose and glycosidic bonds
Differences: Lactulose contains fructose, wheras lactose contains glucose
Lactulose, galactose and monosaccharide X are all reducing sugars. After the lactose has been broken down there is a higher concentration of reducing sugar. Explain why.
2 sugars were produced
Glycogen and cellulose are both carbohydrates. Describe differences between the structure of cellulose molecule and a glycogen molecule
Cellulose is made up of β-glucose monomers and glycogen is made up of α-glucose monomers
Cellulose molecule has a straight chain and glycogen is branched
Cellulose molecule has a straight chain and glycogen is coiled
Glycogen has 1,4- and 1,6- glycosidic bonds and cellulose has only 1,4- glycosidic bonds
What is the test that would be used to show the presence of starch?
Iodine/potassium iodide test
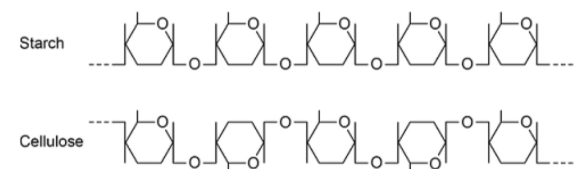
Explain the difference in the structure of the starch molecule and the cellulose molecule shown in the diagram
Starch formed from α-glucose but cellulose formed from β-glucose
Position of hydrogen and hydroxyl groups on carbon atom 1 inverted
Explain how cellulose molecules are adapted for their function in plant cells
Has long and straight chains, becoming linked together by many hydrogen bonds form fibrils, providing strength (to cell wall)
Describe how the structure of glycogen is related to its function
Helix/coiled/branched so compact
Polymer of glucose so easily hydrolysed
Branched so more ends for faster hydrolysis
Glucose (polymer) so provides respiratory substrate for energy (release)
Insoluble so not (easily) lost (from cell)
A precipitate is produced in a positive result for reducing sugar in a Benedict’s test. Suggest a method, other than using a colorimeter, that could be used to measure the quantity of reducing sugar in a solution
Filter and dry the precipitate
Find mass/weight
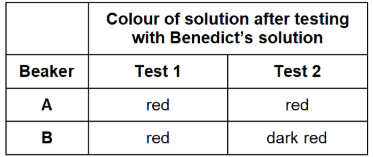
One beaker contained maltose solution and the other beaker contains glucose solution. Both solutions have the same concentration.
Explain the results for beakers A and B in the table
Test 1 – used Benedict’s solution to test for reducing sugar.
Test 2 – added the enzyme maltase, heated the mixture at 30 °C for 5 minutes, and then used Benedict’s solution to test for reducing sugar.
Beaker A: Glucose was present because more sugar
Beaker B: Maltose was present because more sugar after hydrolysis
How would the use of a colorimeter in a benedicts test improve the repeatability of the student’s results
Makes the results Quantitative
Standardises the method
Student used a measuring cylinder to measure 15 cm3 of solution from a beaker. The measuring cylinder gives a volume with an uncertainty of ±1 cm3. She used a graduated syringe to measure 5.0 cm3 of Benedict’s solution. The graduated syringe gives a volume with an uncertainty of ± 0.5 cm3. She mixed these volumes of liquid to do the biochemical test.
Calculate the percentage error for the measurements used to obtain a 20 cm3 mixture of the solution from the beaker and Benedict’s solution. Show your working.
1/15 × 100 = 6.67%
0.5/5 × 100 = 10%
6.67% + 10% = 16.67% (≈17%)
What is an amino acid?
Monomer in a polypetide, it has a central carbon atom bonded to a carboxylic acid group, an amino group, a hydrogen atom and a r group
What is a peptide bond?
Bond between amine group (NH2) of one amino acid and carboxyl group (COOH) of another amino acid
What is a dipeptide?
Two amino acids joined together by a peptide bond
What is a hydrogen bond?
Attraction between a hydrogen atom and an electronegative atom, such as nitrogen or oxygen. It’s weak and is found in the secondary and tertiary structure of proteins
What is an ionic bond for proteins?
If 2 oppositely charged R groups are found close to each other, an ionic bond forms between them
What is a disulphide bridge in proteins?
Where 2 cysteine amino acids are found together, strong covalent bond is formed between the sulphur atoms within the cysteine monomers
What is a primary structure of protein?
Sequence of amino acids in a polypeptide chain, with the sequence being determined by the DNA. Therefore, the primary structure determines the final tertiary structure of protein
What is a secondary strcuture of protein?
The way in which the primary structure of a polypeptide chain folds (2d, forming α helix and β pleated sheets), forming hydrogen bonds that holds its structure (weak bonds between carboxyl of one and amine of another)
What is a tertiary structure of protein?
The final 3d structure of a polypeptide, held in place by hydrogen bonds, ionic bond an disulphide bridges. The disulfide bonds form between the R groups of 2 amino acids that contain sulfur
What is a Quaternary structure of protein?
A protein with more than one polypeptide chain, sometimes they contain a prosthetic group (not made of amino acids) e.g. Haemoglobin contains a haem group
What is a conjugated protein?
Protein with a non-protein group added onto it
What is an enzyme?
Protein that acts as a catalyst and so lowers the activation energy needed for a reaction
What is the active site?
Sequence of amino acids that makes up the region of an enzyme into which the substrate fits in order to catalyse a reaction
What does complementary mean?
The relationship between the active site of an enzyme and the substrate molecule, the way in which they fit together
What is activation energy?
Energy required to bring about a reaction
What is the lock and key method?
Analogy for how enzymes work - only correctly sized key (substrate) fits into the key hole (active site) of the lock (enzyme)
What is induced fit?
Mechanism of interaction between an enzyme and a substrate. As the substrate fits into the active site the active site of the enzyme changes shape in order to allow an enzyme-substrate complex to be formed
What is an enzyme-substrate complex?
The intermediate formed when a substrate molecule binds to the active site of an enzyme
What is an inhibitor?
A substance which reduce sthe activity of an enzyme
What does denature mean?
Change in the structure of a protein brought about by heat or pH, resulting in loss of function
What is metabolism?
All the chemical reactions that take place in living organisms
What are the many ranges of different functions of protein?
Structure, Enzymes, Transport, Pumps, Motors, Hormones, Receptors, Antibodies, Storage, Blood clotting, Lubrication, Toxins and Antifreeze
What is a protein?
Large polymers made up of monomers called amino acids
Give the structure of an amino acid
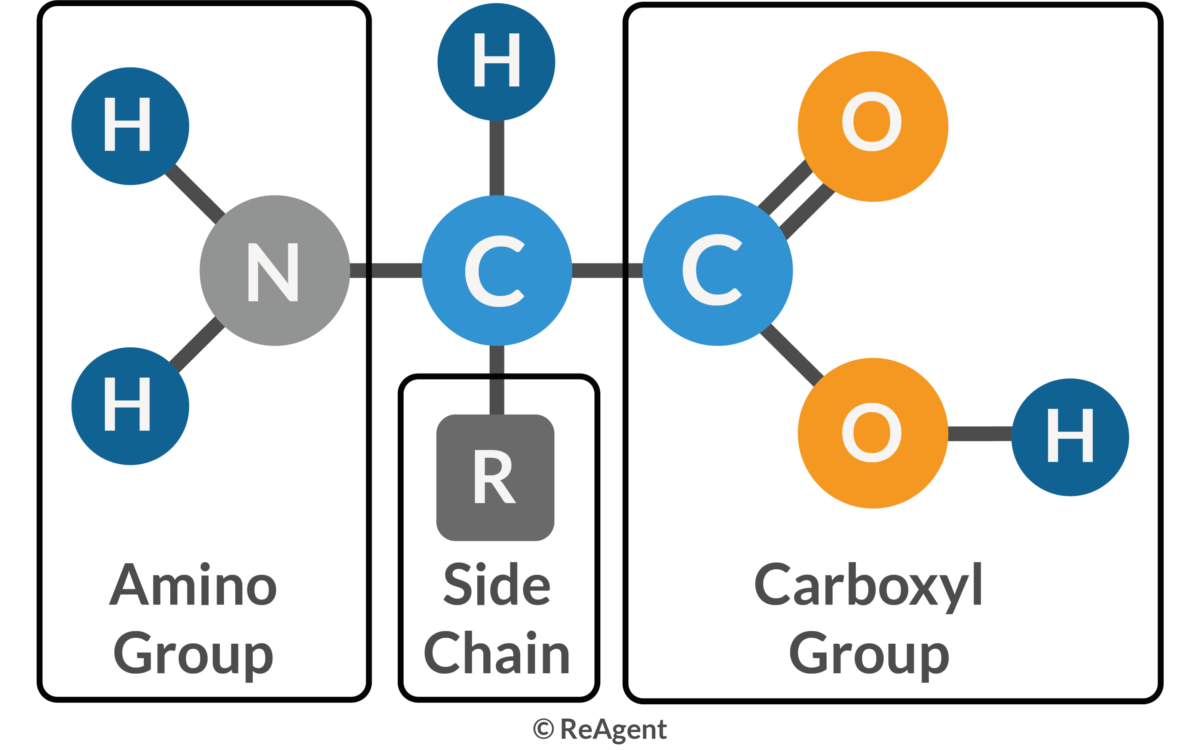
How many amino acids are there with different R groups?
20 different amino acids
Why are amino acids joining together considered a ‘condensation reaction’?
Two molecules join to form a larger one, with water released, which is the definition of a condensation reaction.
What is the N-terminus and C-terminus?
Free group end (sticking out) in a polypeptide, N-terminus is the free NH2 group and C-terminus is the free carboxyl group
Give the summary of the protein structures
Primary - Sequence of a chain of amino acids
Secondary - Local folding of the polypeptide chains into helices or sheets
Tertiary - 3 dimensional folding pattern of a protein due to side chain interactions
Quaternary - Protein consisting of more than one amino acid chain
What do the roles of proteins in organisms depend on?
The protein’s molecular shape, which can be either...
Globular - Carries out metabolic functions e.g. enzymes and haemoglobin
Fibrous - Forms long chains running parallel to each other with cross-bridges between the chains, producing very stable molecules e.g. collagen
What is the structure of collagen in terms of primary, secondary, tertiary and quaternary?
Primary - Unbranched polypeptide chain
Secondary - Polypeptide chain forms an α helix. Lot of amino acid glycine helps close packaging
Tertiary - Polypeptide chain is further twisted
Quaternary - 3 polypeptide chains are wound together with covalent bonds between amino acids of adjacent chains
Explain why the quaternary structure of collagen makes it a suitable molecule for a tendon?
Triple helix structure – Three polypeptide chains are tightly wound together into a rope-like helix, making collagen extremely strong and resistant to stretching.
Hydrogen bonding between chains – The three chains are held together by many hydrogen bonds, which add stability to the structure
Cross-linking between molecules – Collagen molecules are staggered and covalently cross-linked to form fibrils. This gives tendons tensile strength
Parallel arrangement in bundles – Collagen fibrils assemble into fibres that run in parallel along the tendon, perfectly aligned with the direction of force from muscle to bone. This maximises strength in the direction of pull
How do you do the test for proteins?
Add biuret reagent (blue) to a sample of the solution to be tested
if protein is present, solution should turn lilac
What do enzymes do in the body?
Lowers the activation energy as the temperature of the human body is 37oC, which is too low for chemical molecules to react fast enough to support life
What are the enzymes that catalyses metabolic reactions categorised as?
Intracellular - works inside a cell
Extracellular - works outside a cell
Why is a relatively small amount of enzyme able to catalyse a large amount of substrate in total?
They can be reused, they don’t undergo any permanent change in structure as a result of the reaction they catalyse
Enzymes are globular protein molecules, leading to…
Enzymes (and its active site) having a specific tertiary structure and shape, and are very specific to one substrate.
Explain the lock and key model
Model suggests that substrate combines with active site of enzyme (like key with lock). The active site is always the exact complementary shape (at optimum temperature), so reactions are faster in these conditions. Substrate binds to the active site of the enzyme, forming an enzyme-substrate complex. The substrate is broken down and products no longer fit so it leaves the enzyme, with the enzyme unaltered and free to catalyse another substrate. Only issue with this model is that the enzyme (like a lock) is rigid, which scientists believe that the structure is actually flexible.
Explain the induced fit model
More accepted model. Model suggests that a substrate and its active site is not exactly complementary to begin with, but that when a substrate binds with an enzyme, it induces changes in the enzyme structure. The amino acids that make up the enzyme’s active site are moulded into a precise formation to wrap around the substrate to form an enzyme-substrate complex. Active site changes shape to become complementary. This “strained” fit between the active site and the substarte distorts a particular bond in the substrate and so reduces the activation energy needed to break the bond.
Why is the induced fit model a better explanation of enzyme action?
It explains how the binding of other molecules can affect enzymes’ shape and activity, and also provides an explanation for how the activation energy is lowered
How to calculate the rate of reaction?
(Amount of products formed/Amount of reactants used) ÷ Time taken
Enzyme reactions will produce a curve, as the rate will be fastest at the start of the reaction, and get slower as the reaction progresses. Explain why.
More substrates at start
More enzyme substrate complexes
Substrates used up as reaction progresses
What are the requirements for enzyme’s actions to be more effective?
Suitable temperature and pH
Adequate supply of substrate and enzyme
What is pH?
The concentration of hydrogen ions (H+) present in a solution, with a greater concentration of hydrogen ion resulting in a lower pH. The difference in concentration when changing pH is very big
What is the pH scale?
A more convenient way of measuring concentrations of H+ in a solution. pH of 1 has 10-1 mol dm-3 concentration of H+ . pH scale can be said to be logarithmic
How would you calculate the pH of a solution where the H+ concentration is known?
By using the formula:
pH = -log10H+
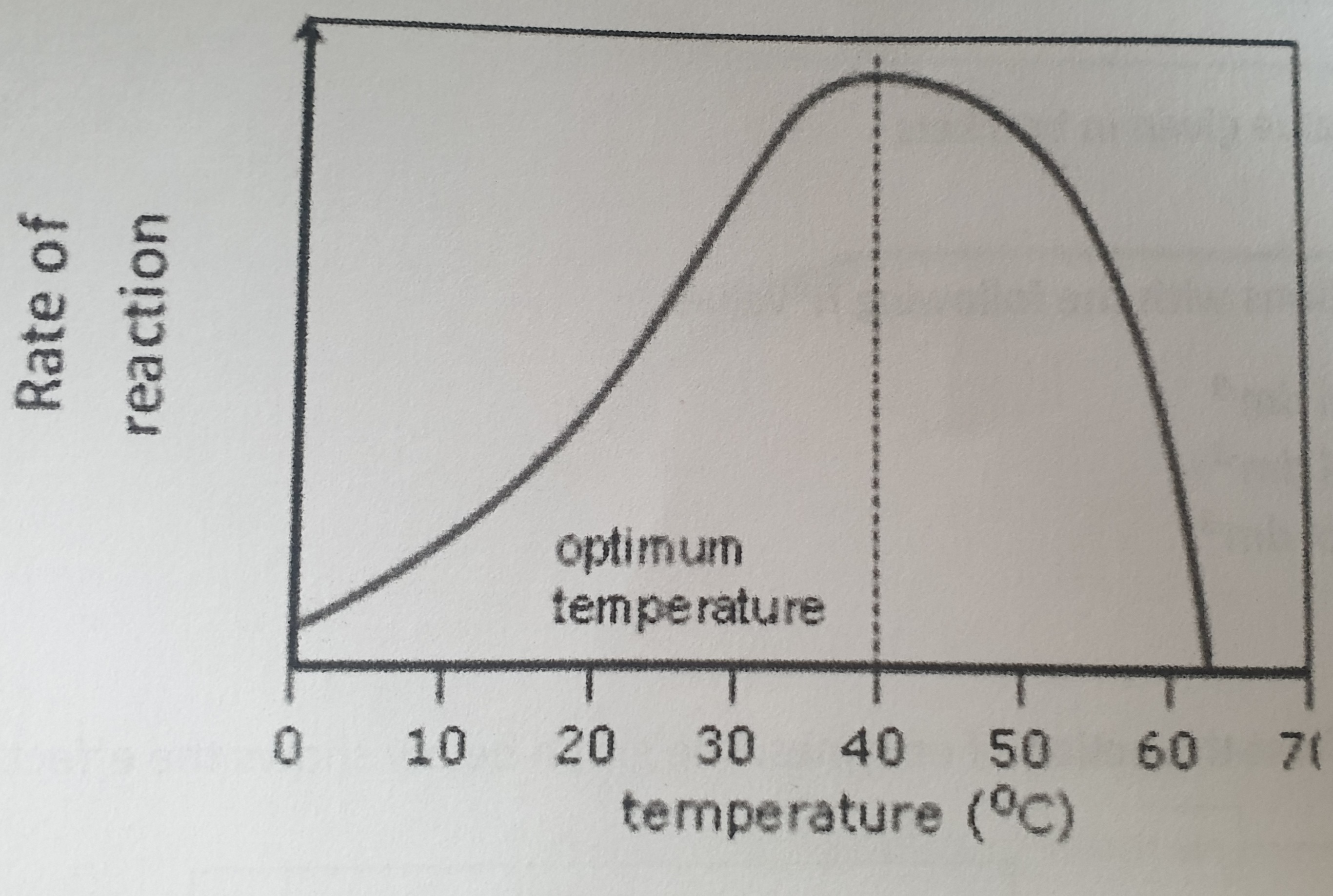
Explain what is happening on this graph.
At very low temperatures, enzymes molecules are inactive but undamaged. As temperature slowly increases, enzyme and substrate move around slowly in aqueous solution, colliding rarely. As temperature gets higher, enzyme and substrate molecules gain more kinetic energy, colliding more frequently and so a greater number of enzyme-substrate complexes are formed and so more product is made, leading to an increased rate of reaction as it reaches the optimum temperature of 40oC
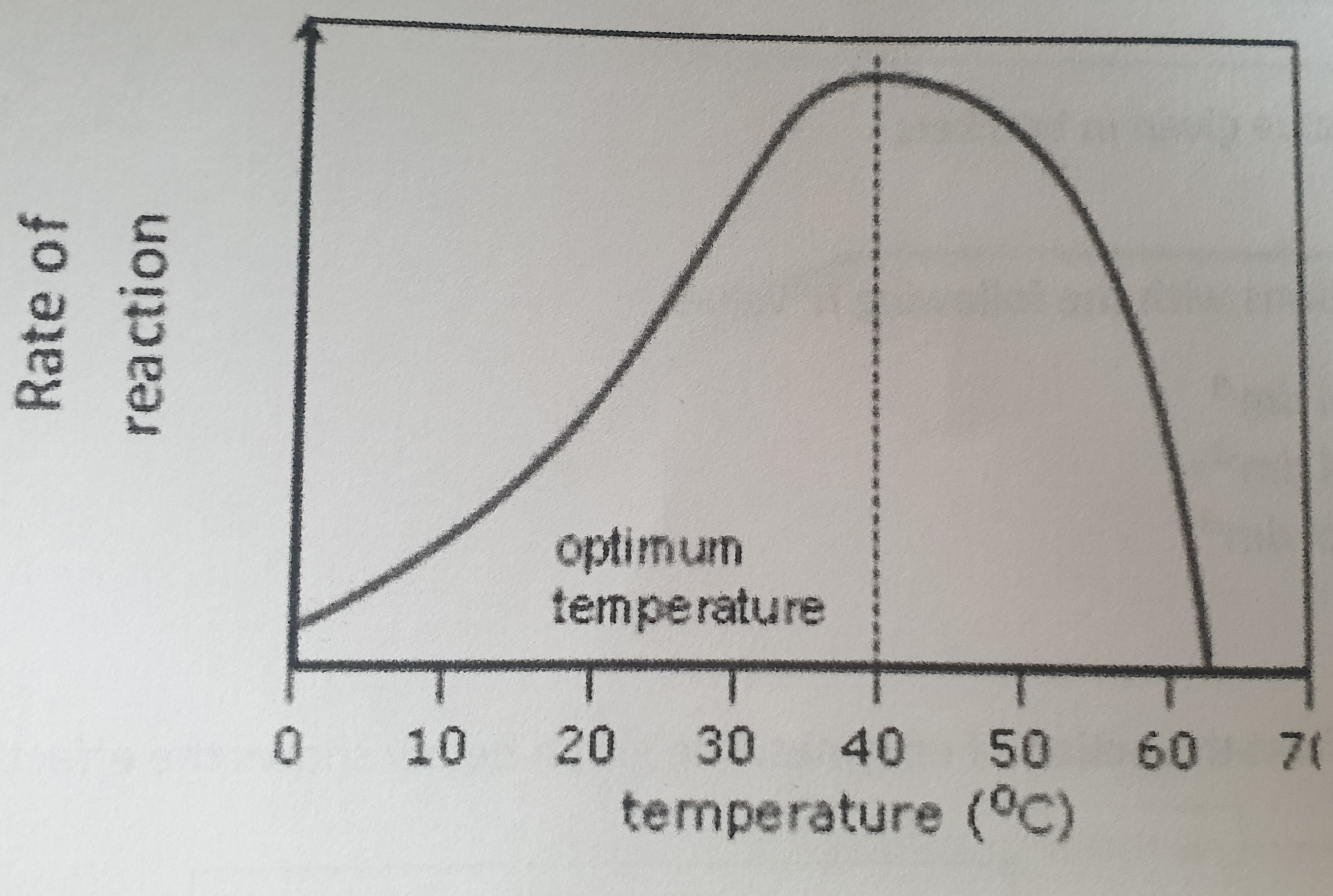
What happens to the enzymes above the temperature of 40oC?
Enzymes are denatured:
H bonds break
Tertiary structure changes
Active site changes shape and substrate no longer fits
ES complexes can no longer form
Higher the temperature the more enzyme molecules will denature
What is optimum temperature?
Temperature at which most molecular collisions occur, yet denaturation barely begins, enzymes works fastest
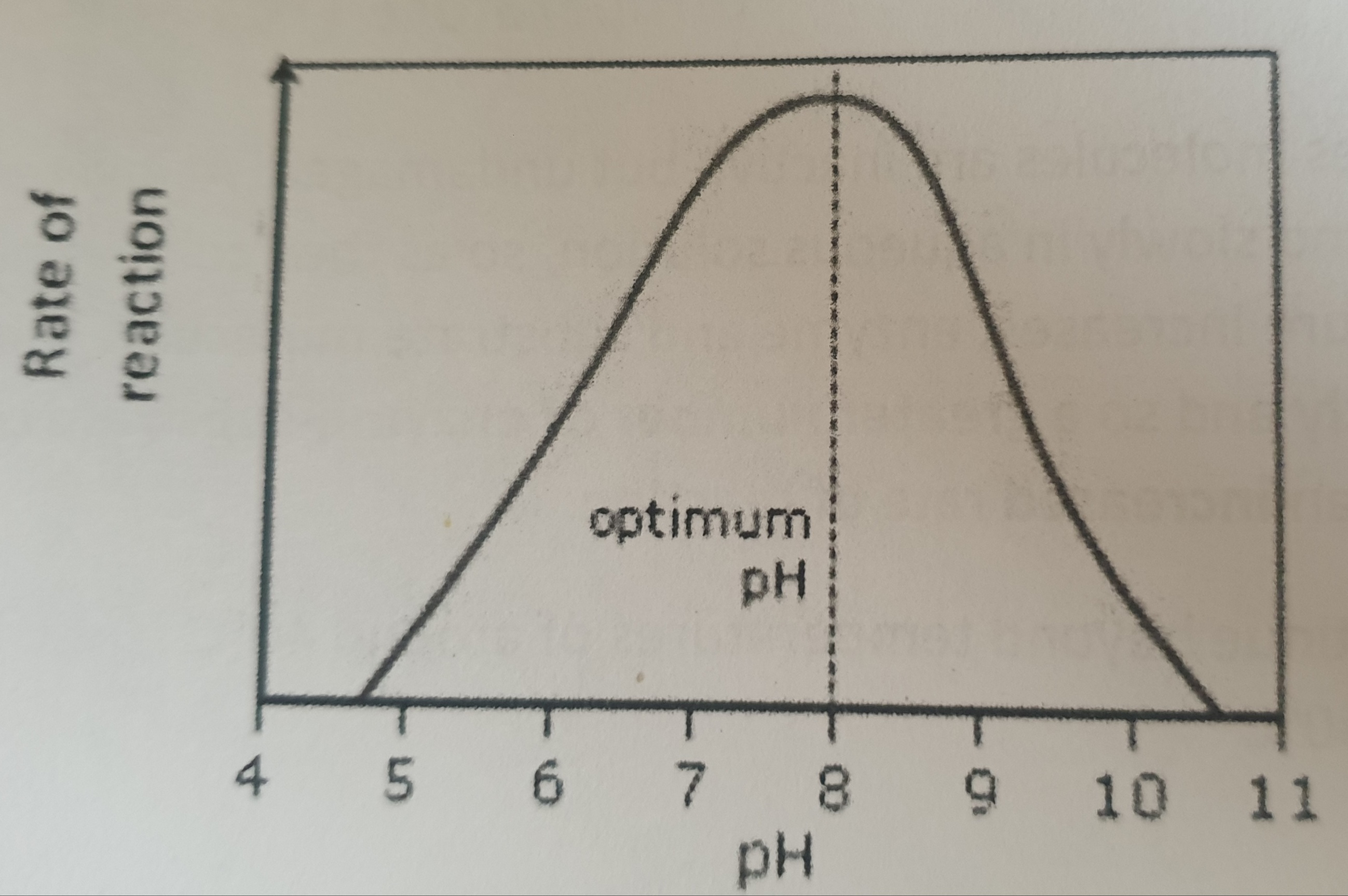
Explain what is happening on this graph.
Change in pH alters the charges on the amino acids which make up the active site of the enzyme, Causing some of the hydrogen and ionic bonds that maintain the enzyme’s tertiary structure to break and reform in different places. These changes can alter the shape of the active site and the substrate may therefore no longer fit. Therefore, no enzyme-substrate complexes can be formed. The enzyme has been denatured.
Why do enzymes work best at the optimum pH?
Active site is exactly complementary to the substrate, so maximum number of enzyme substrate complexes form
Many enzymes have an optimum ph of around 7, Give an example of an enzyme with a more extreme optimum pH?
Peptidases, optimum around pH2