Reactivity 3.3: Organic Chemistry~hydrocarbons
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
Define Homologous Series
A series of compounds of the SAME family, with the same general formula, which differ by a common structural unit
successive members differ from each other by −CH2−. group
They show a gradual change in physical properties (for example their melting-/boiling points increase as a result of increasingly stronger London dispersion forces).
What are Alkanes? What is its Formula
Homologous series of SATAURATED hydrocarbons containing carbon-carbon single bonds
Formula: CnH2n+2
What are Alkenes? What is its Formula?
Homologous series of UNSATURATED carbon-carbon-carbon double bonds
Formula:CnH2n
What are Alkynes? What is its Formula?
Homologous series of UNSATURATED carbon-carbon-carbon triple bonds
Formula:CnH2n-2
What are Aromatic Compounds
Contain at least 1 benzene ring
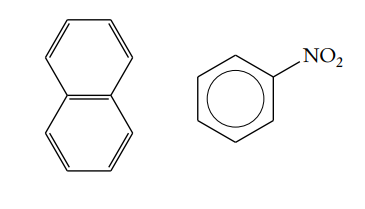
What are Aliphatic Compounds
Compounds WITHOUT benzene rings
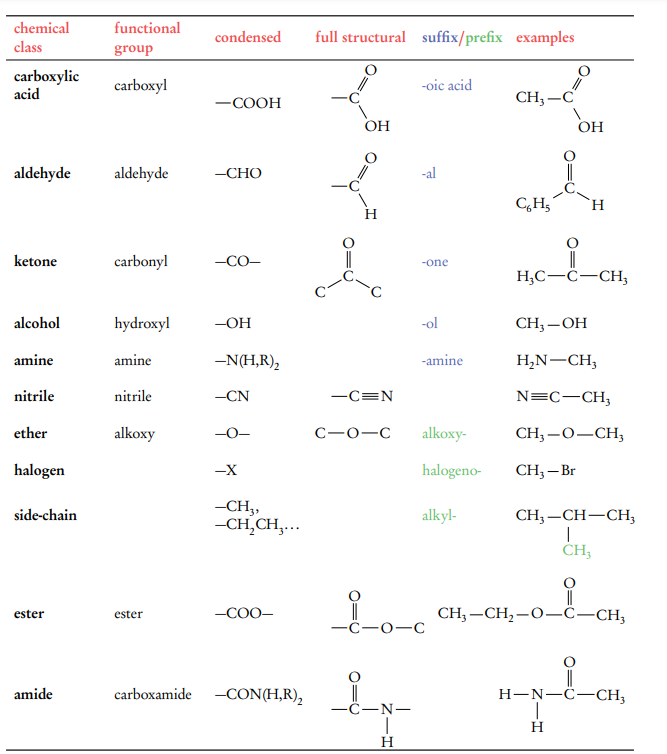
Be able to Identify Functional groups and structures
Define Homolytic bond fission
A covalent bond between 2 atoms in a molecule breaks with each atom taking 1 electron from the bond
Results in free radicals, which are HIGHLY reactive as they are unpaired
Define Heterolytic Bond fission
Covalent bond between 2 atoms in a molecule breaks with on atom taking both bonding electrons
Results in cation/anions
Why do Alkanes have LOW reactivity
Due to strong C-C and C-H bonds and they are non-polar which makes them unreactive to common(polar) reactants
What reaction do alkANES undergo?
Free radical substitutions (halogenation)
Combustion
Formula for Complete Combustion
CxHy + O2 —> CO2 + H2O
When oxygen supply is sufficient
Formula for Incomplete Combustion
CxHy + O2 —> CO + H2O
When Oxygen supply is limited
Carbon is sometimes produced in extreme conditions
What are the 3 processes Alkanes go through to undergo halogenation
Initiation
Propagation
Termination
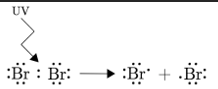
Describe Initiation
UV light is needed to break the bond through homolytic fission to produce free radicals
Describe Propagation
Series of rxn between radicals to produce non-radicals and free radicals
the number of free radicals stays the same
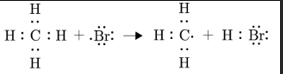
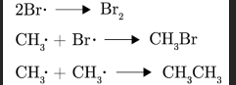
Describe Termination
Free radicals react with each other to form molecules and reaction stops when there are no more radicals
Number of free radicals decreases
Why do alkenes have higher reactivity than alkanes
Because double bonds are the site of reactivity
pi bonds have electron density farther nuclei, so it is more easily broken
What reaction do AlkENES
Electrophilic addition rxn, where 2 molecules combine to produce larger molecule
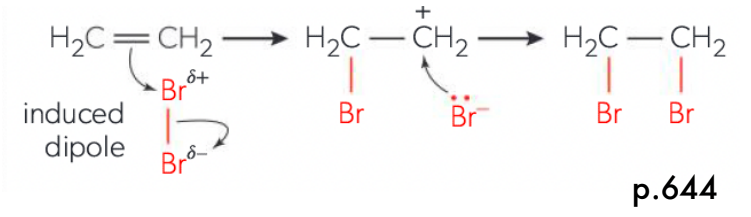
Describe the steps of electrophilic addition steps
Molecule being added (e.g. halogen) NEEDS to be polarized(slightly (+), slightly (-)). Allows electrons in molecule to be repel electron-rich double bond as molecule approaches it
Double bonded carbons is attached by atom with partial positive charge, and the molecule splits HETEROLYTICALLY (creates a negative atom).
Positive atom is attached and adjacent carbons become + charged (carbocation intermediate)
Unstable carbocation and anion form a bond creating a stable product
what reactions do alkenes undergo
Hydrogenation (hydrogen gas reacts with alkene)
Hydration
Halogenation(Alkenes react with halogens to produce disubstituted halogenoalkanes)
Hydrohalogenation
Describe hydrohalogenation (symmetric addition)
Hydrogen halides react with alkenes to produce monosubstituted hydrogenoalkanes
H—X is polar molecule that undergoes heterolytic fission as well to form H+ and X- . Therefore molecule does NOT need to be polarized first
H+ (electrophile) attacks double bond, then carbocation intermediate reacts with X-.
Reactivity of hydrogen halides: HI>HBr>HCl(bonds increases in strength as reactivity decreases)
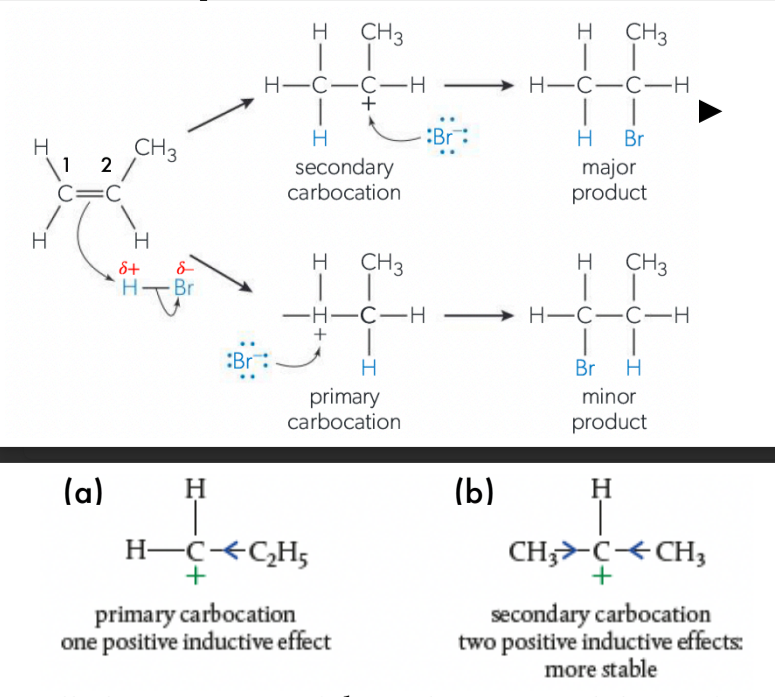
Describe asymmetric addition reaction
When an asymmetric alkene (such as propene CH2 −−CH−CH3 ) reacts with an asymmetric reactant (such as H X or H OH), two structural isomers are formed from two different competing reactions.
According to Markovnikov’s rule: the + δ H or + δ X gets attached to the carbon with more hydrogen substituents, and the halide − δ X attaches to the carbon with more alkyl substituents (more stabilised carbocation aka secondary carbocation).
Describe hydration
Reaction between water and alkene to form alcohol
Uses heat and concentrated sulfuric acid as catalyst
This electrophilic addition produces wither 1 or 2 products (depending if alkene is symmetric or asymmetric)
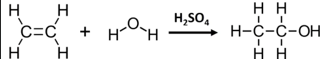
Describe hydration (electrophilic addition of water) steps
The double C=C bond is broken because it is attacked by H+ present in sulfuric acid, which converts into a C-C single bond, making a carbocation.
Water then acts as nucleophile(electron-rich) and attacks the carbocations, forming the alcohol
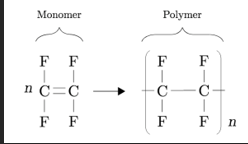
Describe polymerization of alkenes
Formed when smaller unsaturated molecules aka alkenes (monomers) react together
In addition polymerization, small monomers that contain a C=C double bond link together to form a longer polymer
During the process the double bonds in the monomers are converted into single bonds in the polymer