Chapter 18 - ketones and aldehydes
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Ketones
R - C (= O) - R

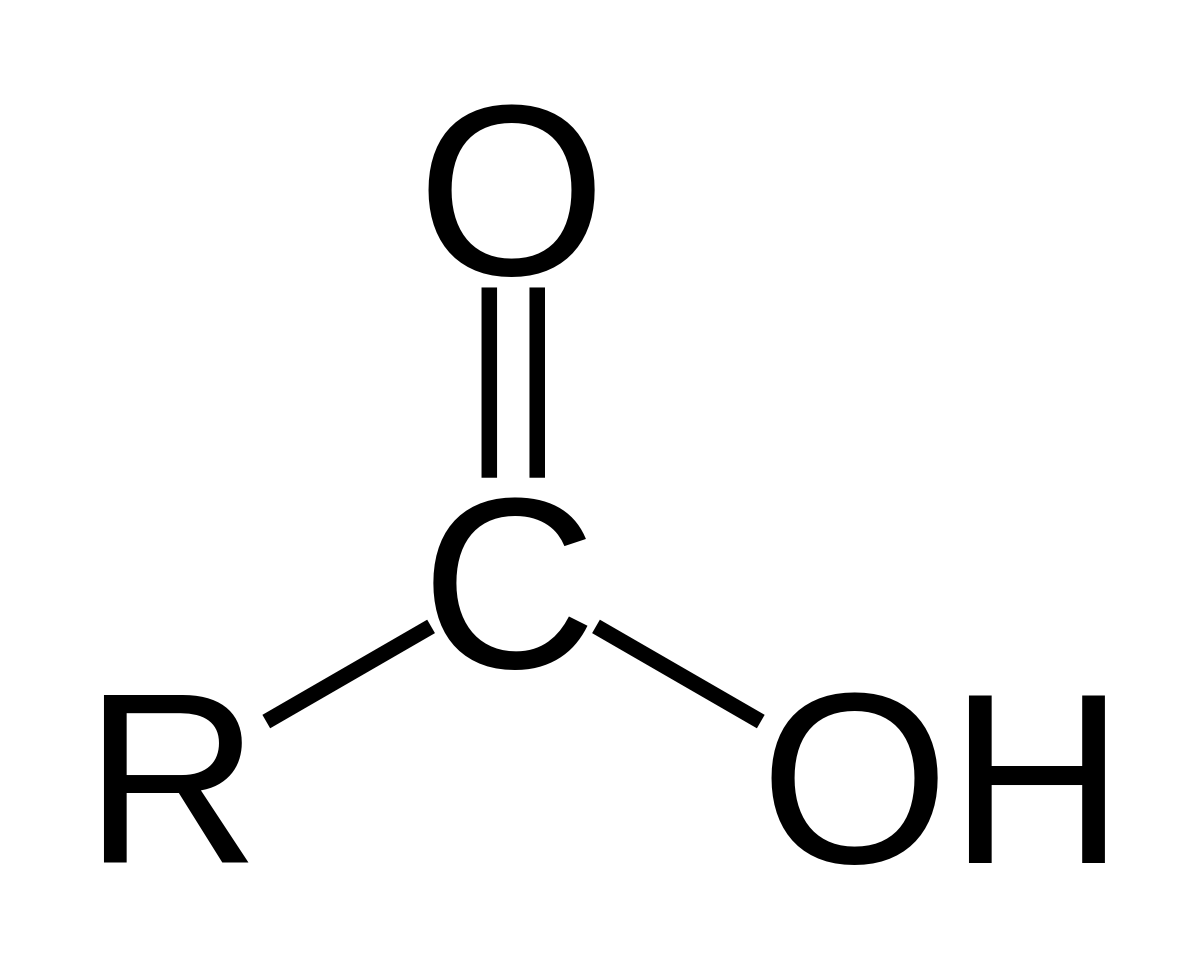
Carboxylic acid
R - C ( = O) - OH
Ester
R - C ( = O ) - O - R

Aldehydes

Acid Chlorides

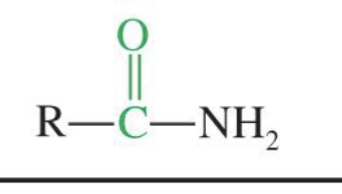
Amides
Structure of carbonyl group
SP2 hybridized
the C = O is shorter and stronger than more polar the C = C Bond in alkene
solubility of ketones and aldehydes
Good solvent for alcohols
The oxygen in the carbonyl group can form hydrogen bonds with O—H or N—H groups.
Small ketones and aldehydes (like acetone and acetaldehyde) mix completely with water.
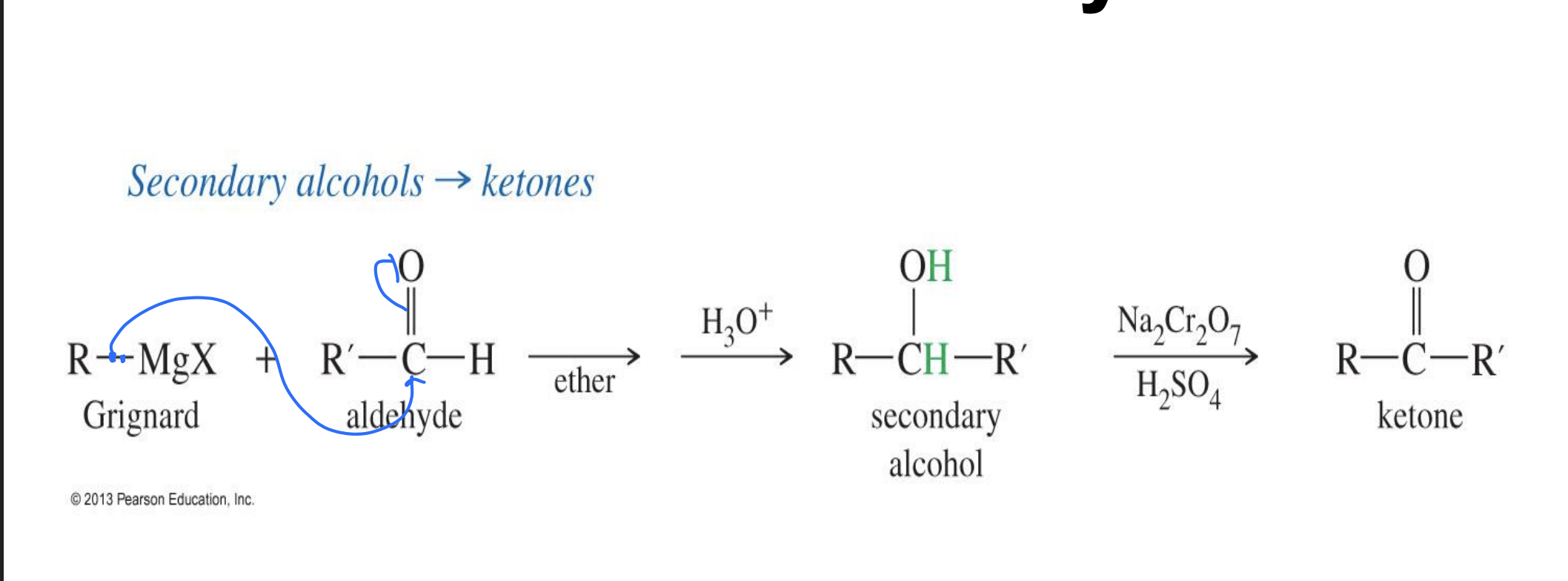
Grignard reagent
Reagent :
R - mgX
H30
Na2Cr2O7, H2SO4
Starting
with an aldehyde
Mechanism
the R - MgX attacks the double bond of O and the R group attaches and MGX leaves.
H30 (acid ) give an H to the O making it in alcohol
Na2Cr2O7 takes the Hydrogen of the alcohol making it a ketone
End product:
ketone
Oxidation of primary alcohols to aldehyde
Starting product: primary alcohol
Reagent:
PCC (Pyridinium chlorochromate)
swern
DMP
PCC is used to oxidize primary alcohol to aldehydes
End product: aldehyde
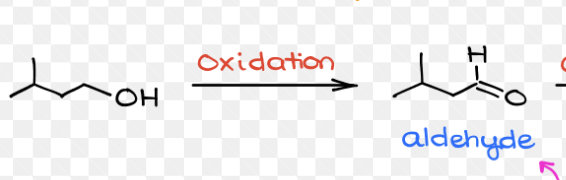
Ozonolysis Of alkenes
Reagent:
O3 ,
Reductions (CH3)2S (DMS)
Starting product: Alkenes
Brakes the double bond followed by a reduction
End Product: Ketones and Aldehydes
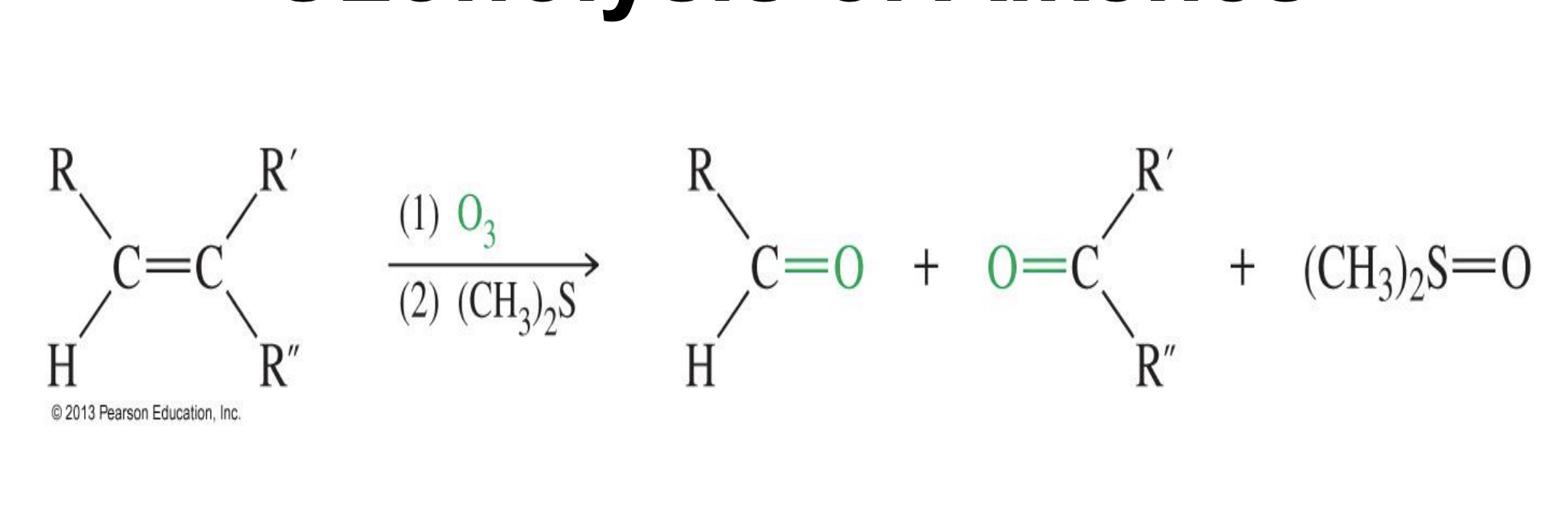
Friedel craft reduction
Starting: Aromatic ring
Reagent:
Acid halide (RCOCl)
Lewis Acid (AlCl₃)
Steps
Lewis acid takes the Halide from R-C(=O)-CL
Carbocation is formed and benzene attacks it and then its added on
Reaction between an acyl halide and an
aromatic ring will produce a ketone.
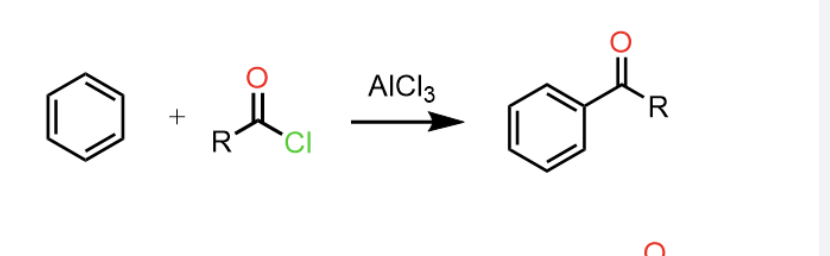
Reduction of Nitriles to Aldehydes
Starting product: nitrile (R - C≡N)
Reagent: (i - Bu)2 ALH → aluminum hydrides
or DIBAL - H
H3O +
Convertes nitriles to aldehydes
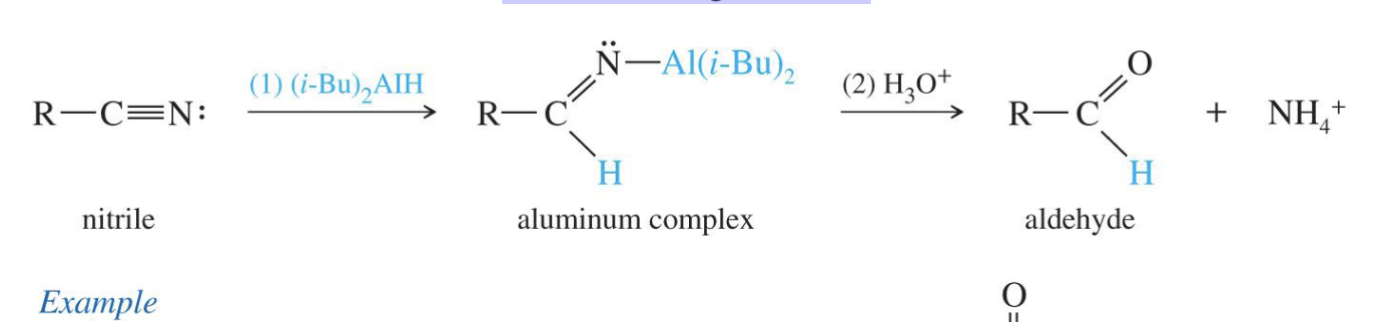
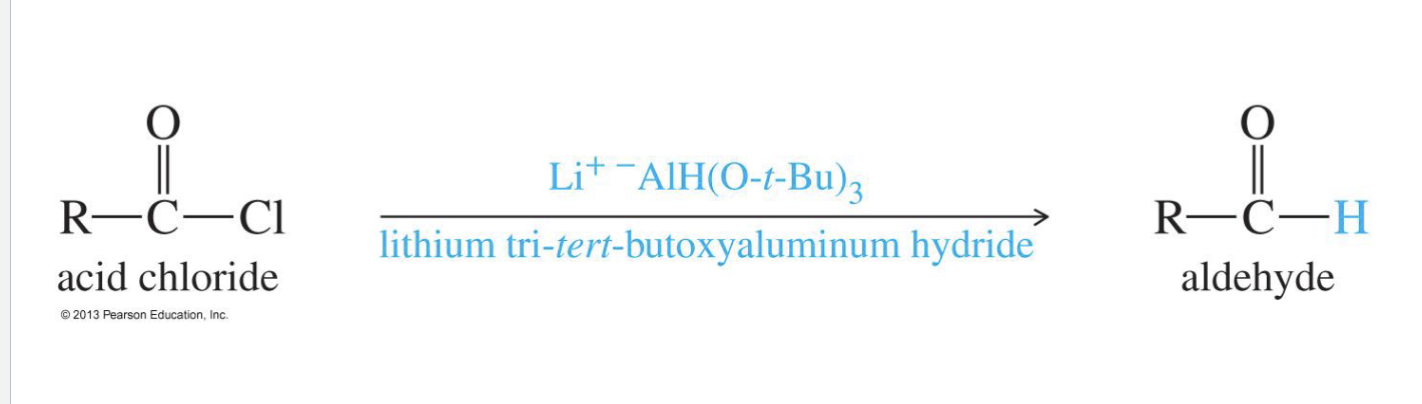
Aldehydes from acid chloride
Starting Product:
Acid chloride ( R - C (=O) - Cl
Reagent:
Li+ -AlH(O t-but)3 → Lithium aluminum tri(t-butoxy)hydride
End product: aldehyde
Reactivity Aldehydes vs Ketones
Aldehydes are more reactive then Ketones
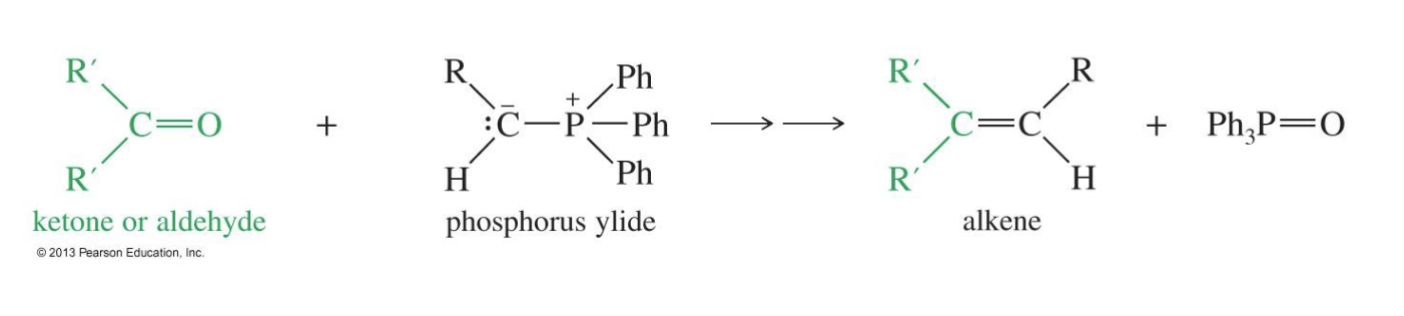
The Wittig Reaction
Starting product: Keton or aldehyde
Reagent: Phosphorus ylide
Converts a carbonyl group into a new C=C double bond
Phosphorus yield is used a the nucleophile in the reaction
Mechanism:
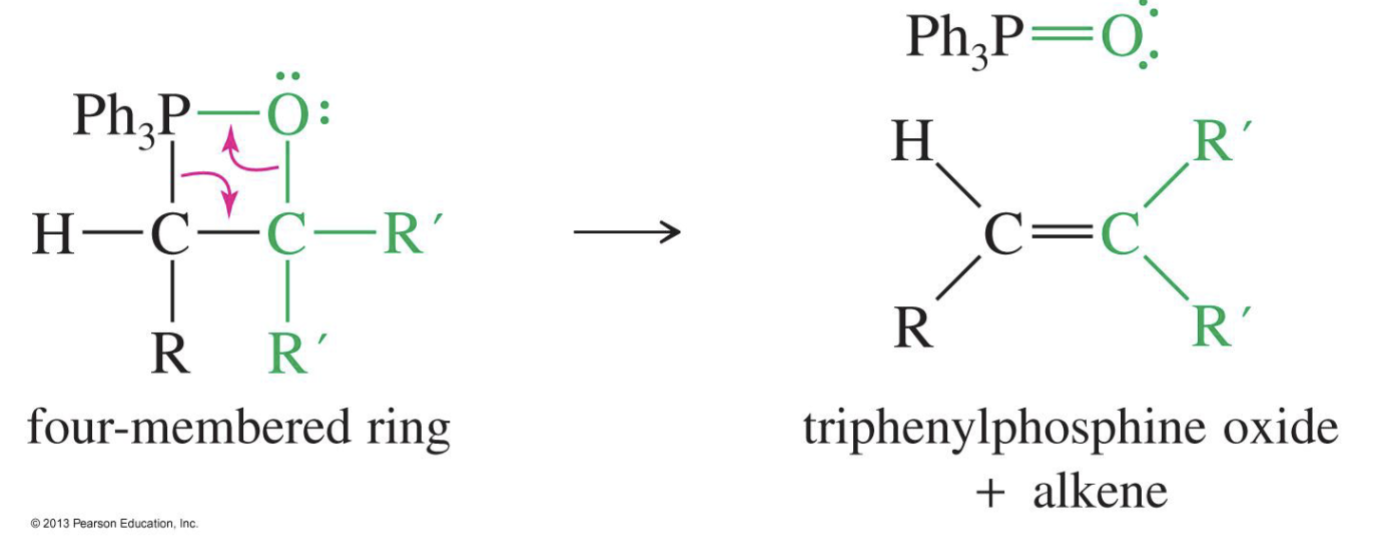
the Phosphorus ylide attacks the keton or aldehyde
the double bond of O breaks and it is left as negative O- (betaine)
The O forms a bond with the adjacent Ph3P (Oxaphosphetane formation)
but this will collapse and the Oxygen will break its bond with carbon and leave with Ph3P and the carbon will become a double bond and then form a carbonyl (ketone or aldehyde ) see down below.
SN2 mechanism
Preparation of Phosphorus Yield (Wittig reaction)
Starting:
triphenylphosphine
Reagent:
alkyl halide R - C - X
Basea:
n-Butyllithium (n-BuLi)
Sodium hydride (NaH)
Potassium tert-butoxide (t-BuOK)
Mechanism
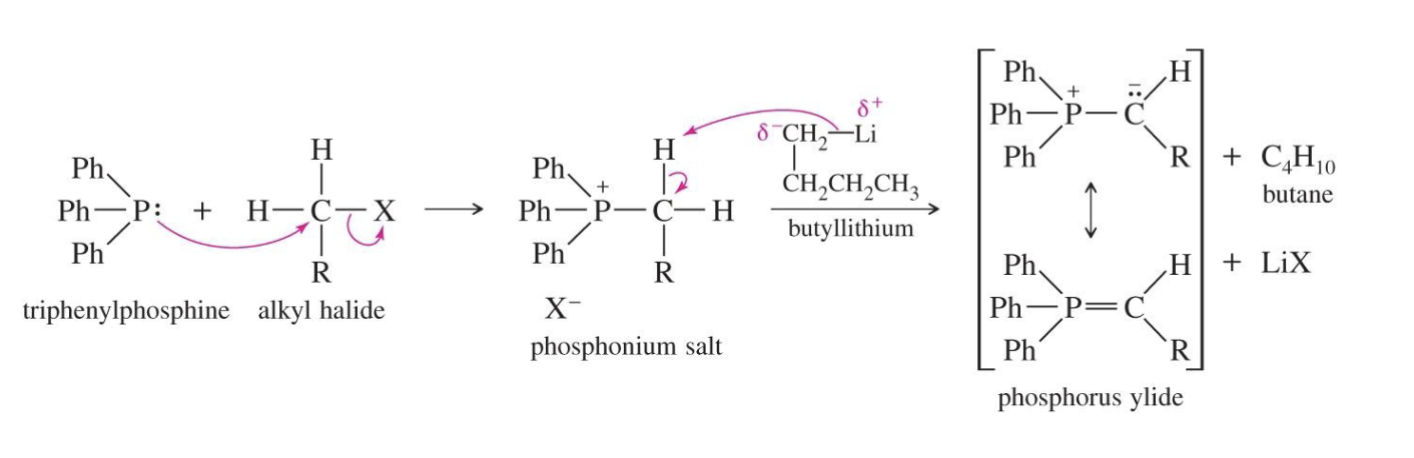
triphenylphosphine attacks Alkyl halide
the triphenylphosphine kicks the X (halide) out and a new bond is formed
The base takes a hydrogen from carbon.
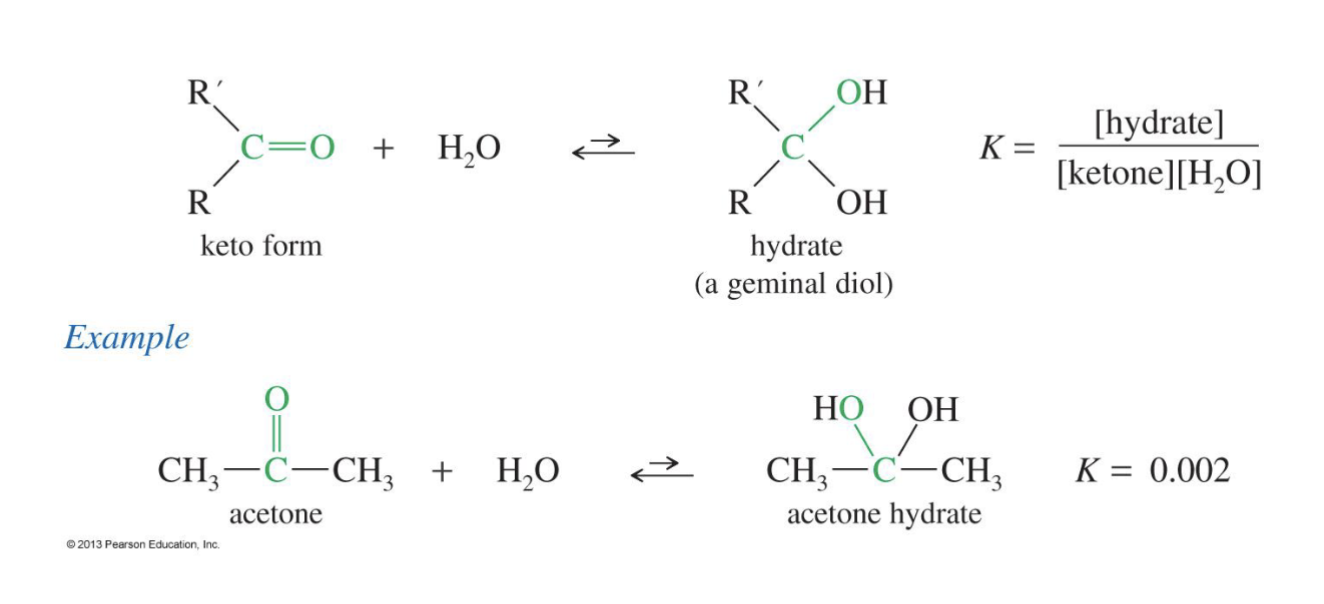
Hydration of Ketone + Aldehyde
Reagent:
H2O
Starting Product
Ketone
Aldehyde
End product:
2 Alcohol group attached to the carbon where the double bond of Oxygen was originally
geminal diol
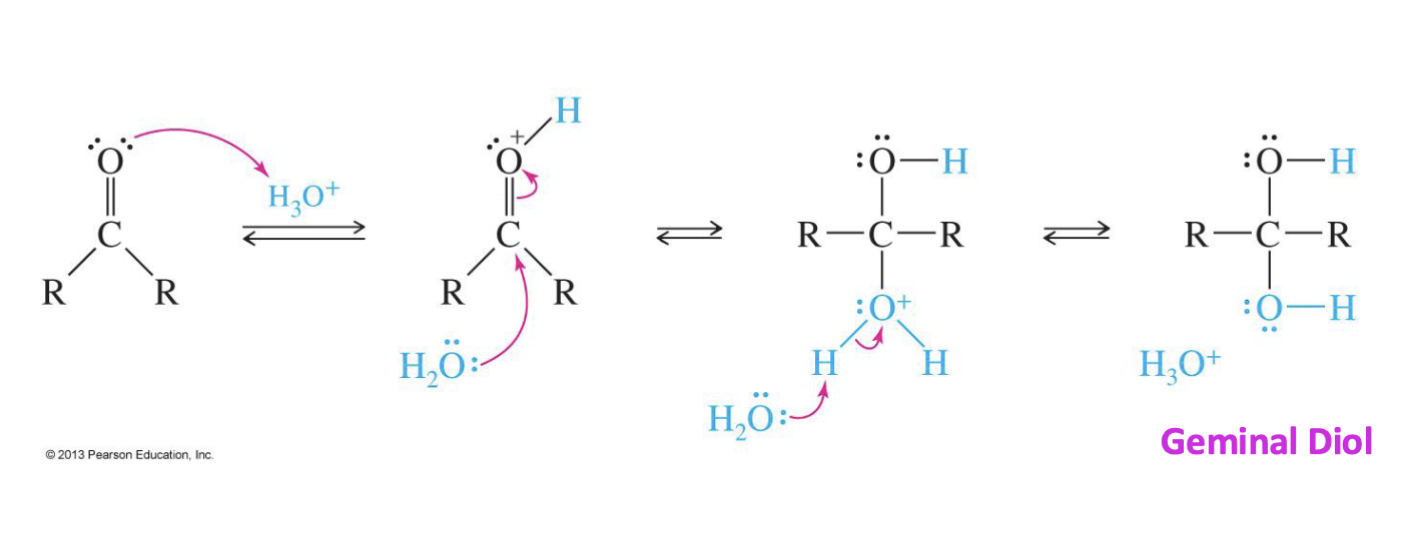
Acid-Catalyzed Hydration of Carbonyls
Reagent:
H3O
H2O
Starting product
Ketone or
Aldehyde
Mechanism
H3O give a hydrogen to O (oxygen)
Water (H2O) comes in and attacks carbon
Another water comes in and protonates ( The H that was from the water)
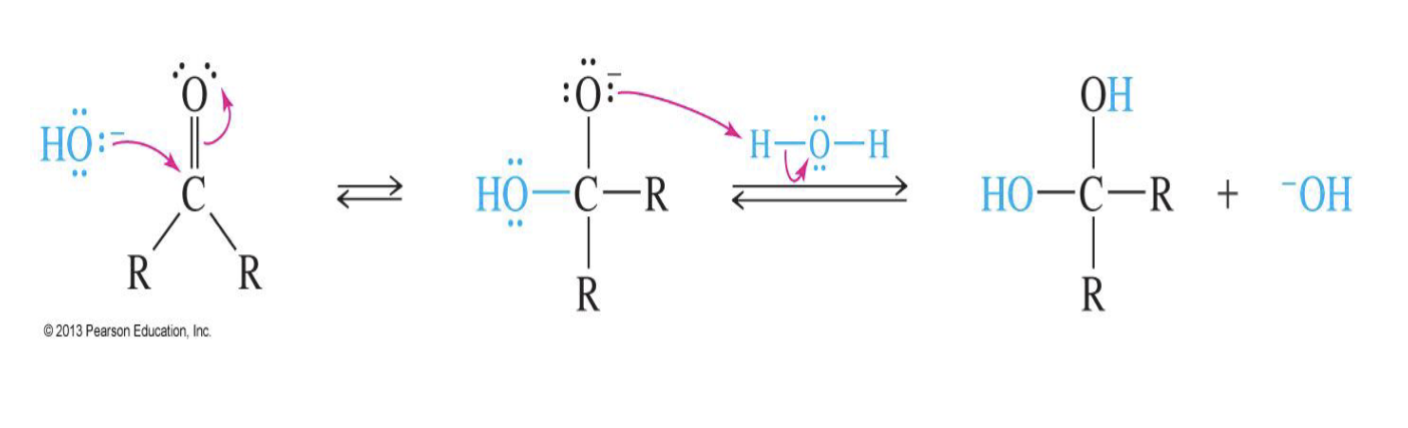
Base-Catalyzed Hydration of Carbonyls
Reagent:
Hydroxide ion (OH-)
H2O
Starting product:
Ketone
Aldehyde
Mechansium:
the base (OH ) attacks Carbon
the double bond breaks making O-
Water comes in and Oxygen takes a hydrogen from water
End product
two –OH groups on the same carbon (geminal diol)
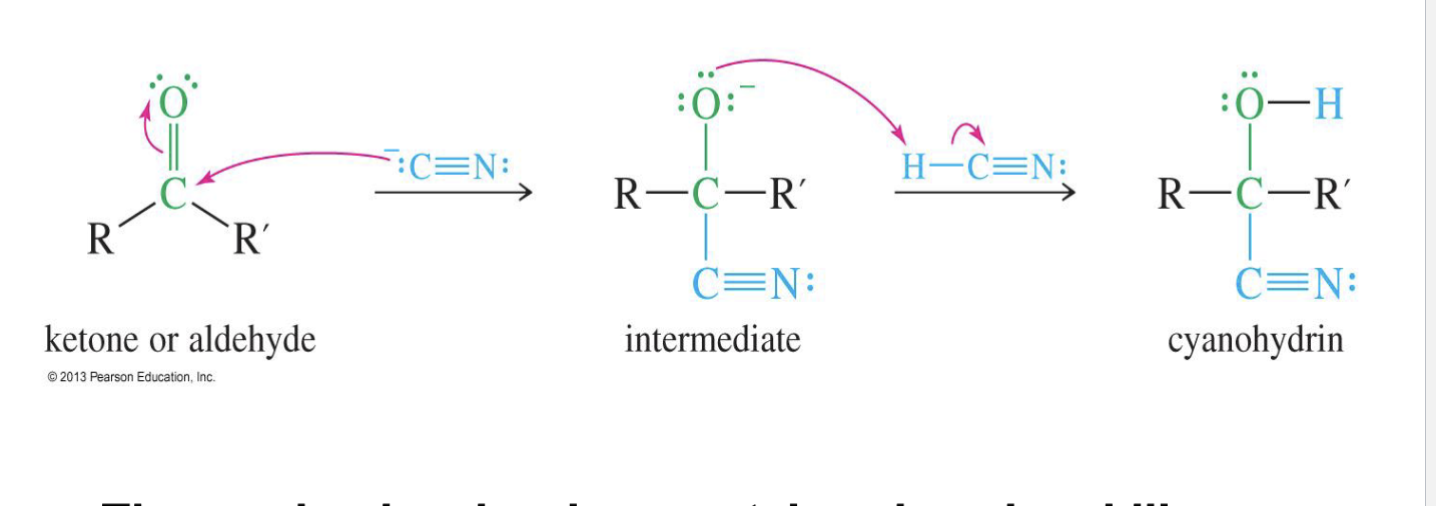
Cyanohydrin Formation
Reagent
-:C≡N (Hydrogen cyanide)
can also be seen as NaCN
H - C≡N
Starting product
ketone
Aldehyde
Mechansium
The CN⁻ attacks the carbon of the carbonyl (C=O)
The oxygen from the carbonyl grabs a proton (H⁺) from HCN or water.
End product:
cyanohydrin (–OH and –CN attached to the same carbon).
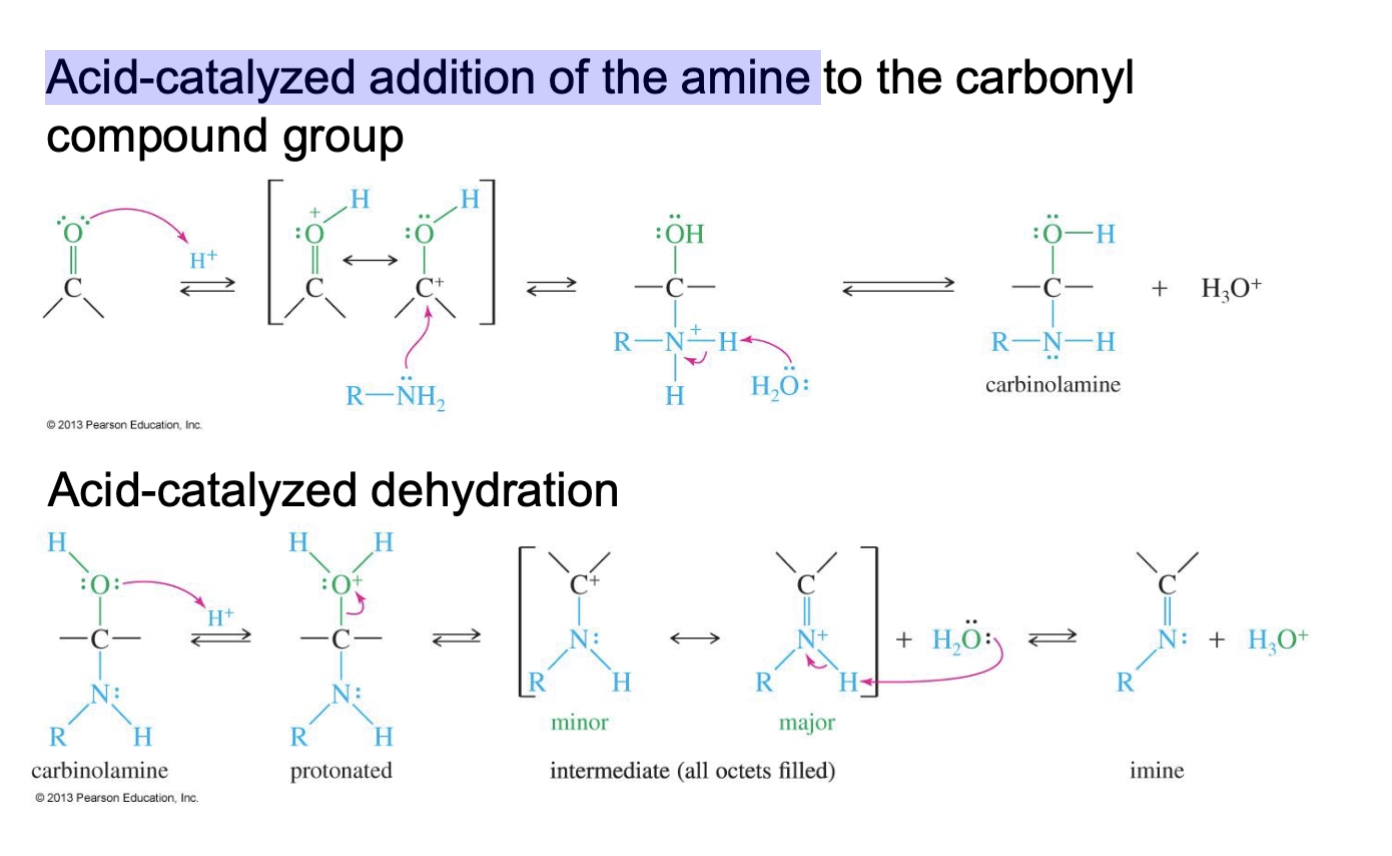
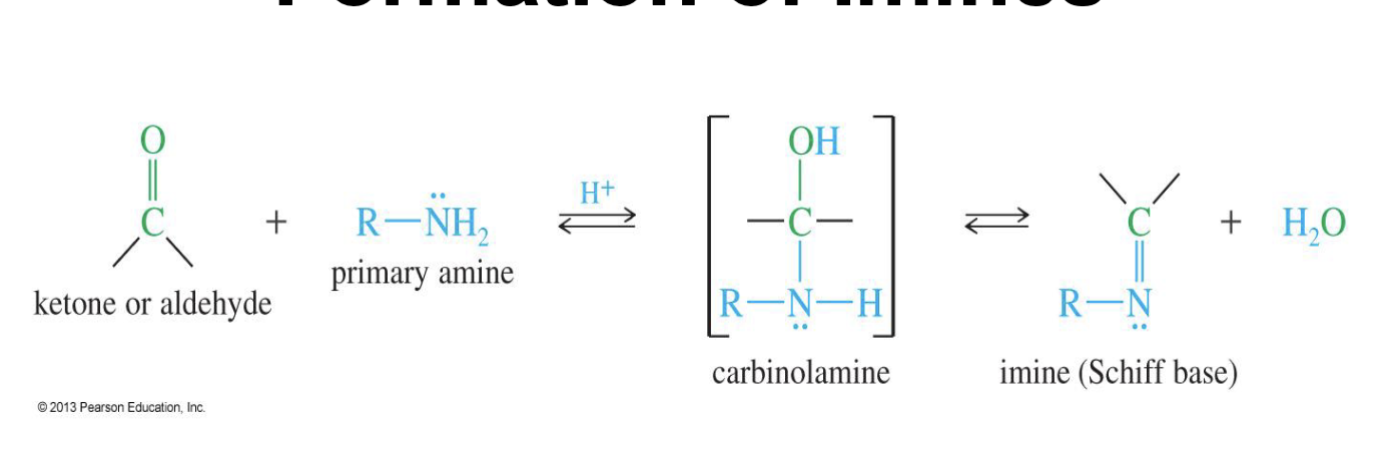
Formation of Imines
Reagent :
1 amine or ammonia (R - NH2)
H+
Starting: Ketone/Aldehyde
Mechanism:
Step 1: Acid-catalyzed addition
The hydrogen form the acid from H+ is taken by Oxygen.
the double bond of of O breaks
The nitrogen of the amine attacks the carbonyl carbon.
Water comes in and takes an H from RNH2 making it R- NH.
Step 2: Acid-catalyzed dehydration
Another H+ is introduces and Oxygen taking a H makes it a L.G H2O
A double bond with carbon and nitrogen from
Water comes in and take an H from R- NH
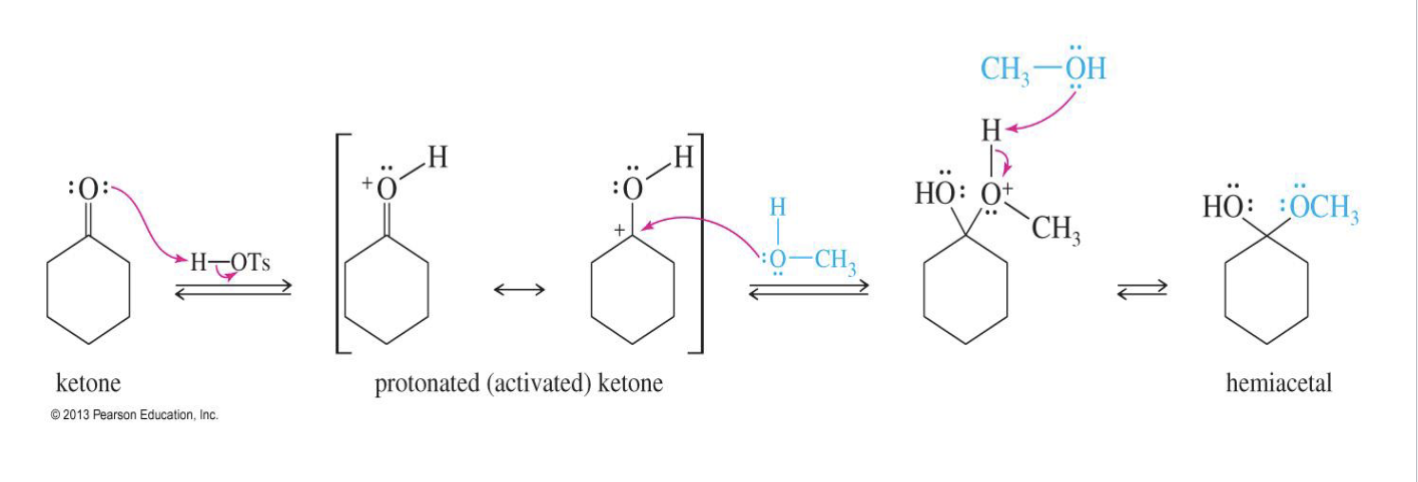
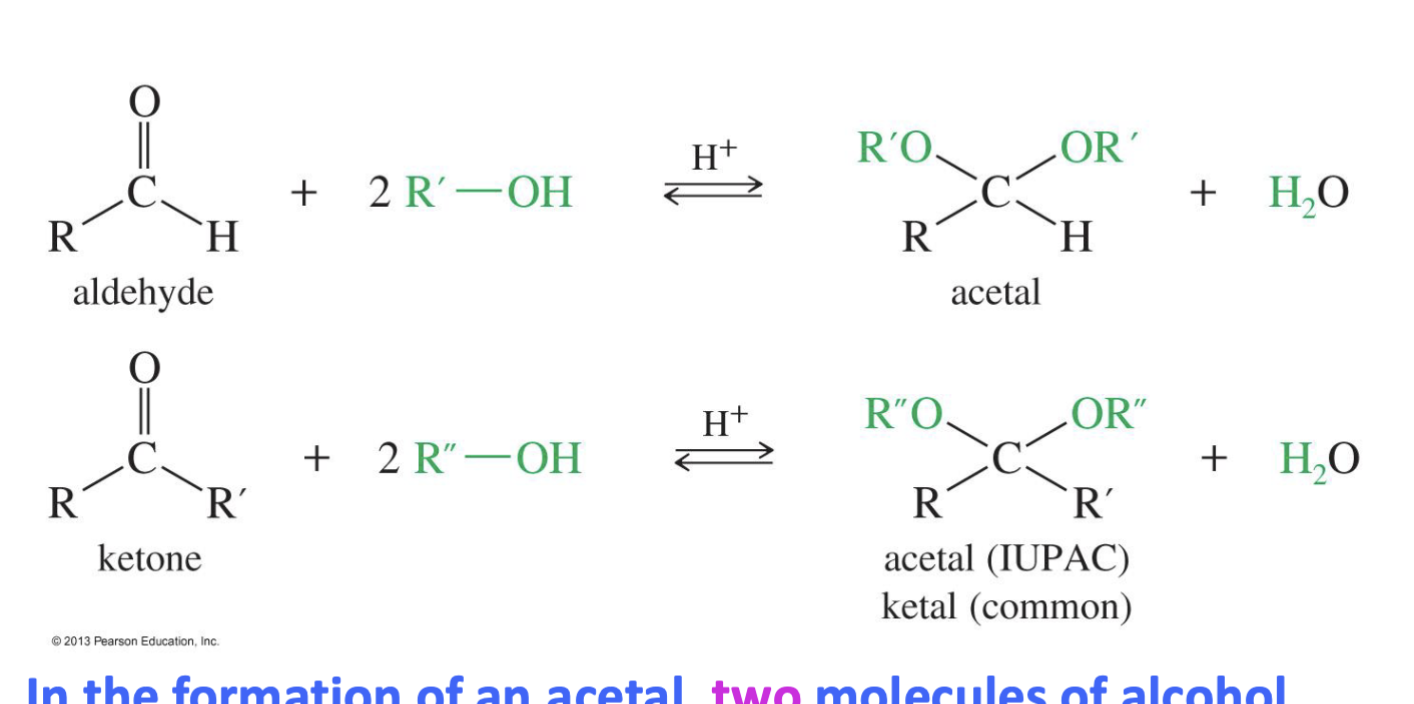
Formation of Acetals
Reagent
2 R - OH
H+ (H - OTs)
Starting
aldehyde
Ketone
Mechanism:
Step 1: Carbonyl oxygen gets protonated (this is before the alcohol attacks).
Step 2: Alcohol attacks the carbonyl carbon → tetrahedral intermediate → hemiacetal.
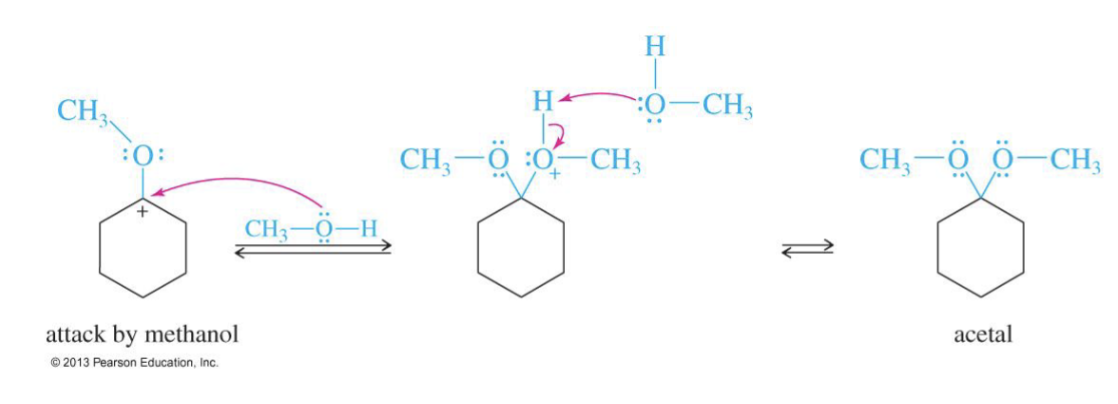
Step 3: Another H+ and Oxygen takes another hydrogen make it H2) and it leaves
Step 4: Second alcohol attacks → acetal formed.
another alcohol comes in and takes a hydrogen
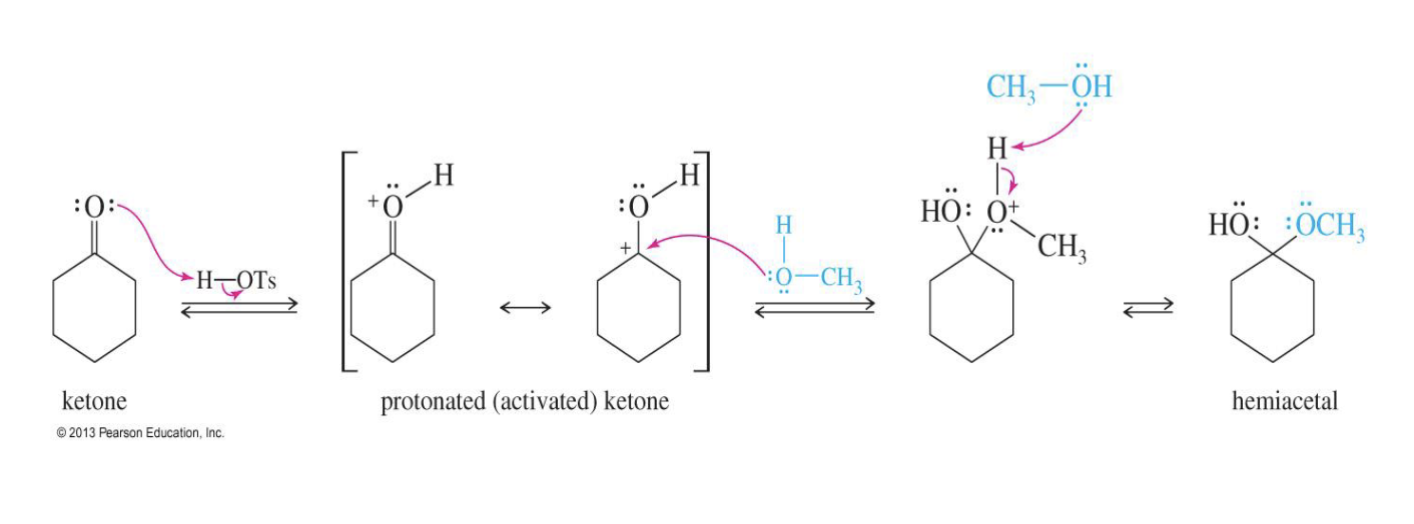
Hemiacetal Formation (intermediate step)
Starting:
Ketone/ Aldehyde
Reagent:
H+
R- OH
Mechanism:
The oxygen takes an H from H+
the first alcohol comes in and attacks the carbon
another alcohol comes in a takes the hydrogent from the first alcohol.
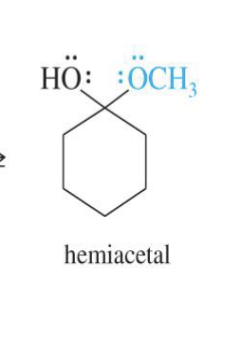
Hydrolysis of Acetals
Starting product:
Acetal
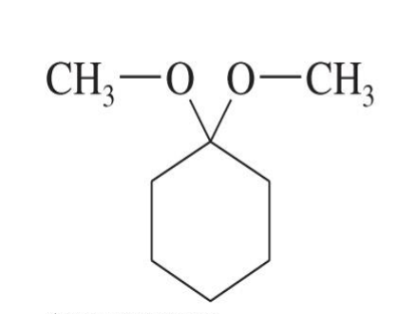
Reagent:
Water (H2O)
Acid catalyst (H⁺)
End product:
The original aldehyde or ketone
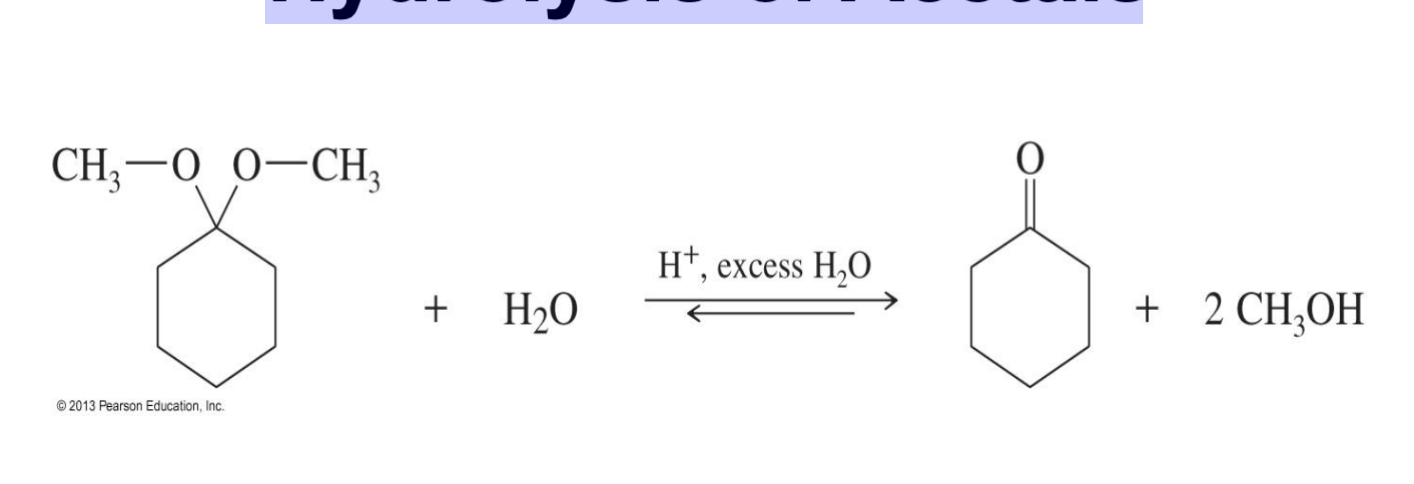
Cyclic Acetals
Starting
Aldehyde or Ketone (R–C=O)
Reagent:
H+
Diol
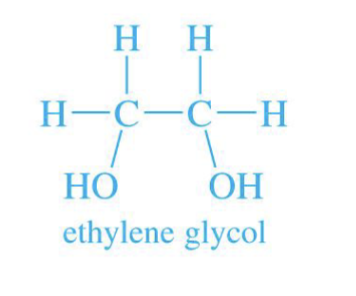
A diol reacts with a carbonyl to make a ring-shaped (cyclic) acetal.
The reaction can go backward (it’s reversible).
Chemists use this to protect aldehydes or ketones during other reactions.
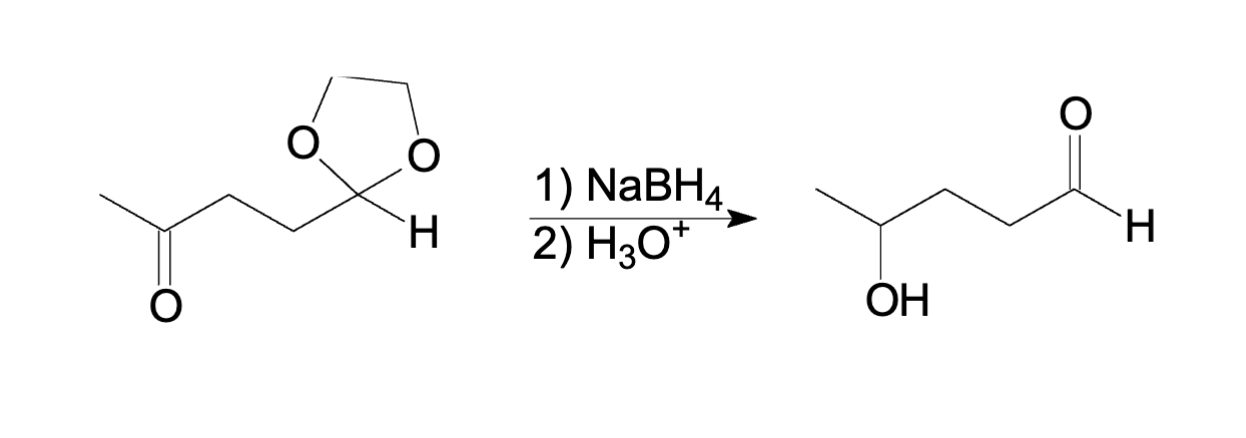
Acetal with NaBH4
Acetal will not react with NaBH4
so is this example only the ketone will reduced
and the Acetal will experience hydrolysis and will protonate the alcohol and remove the acetal to aldehyde
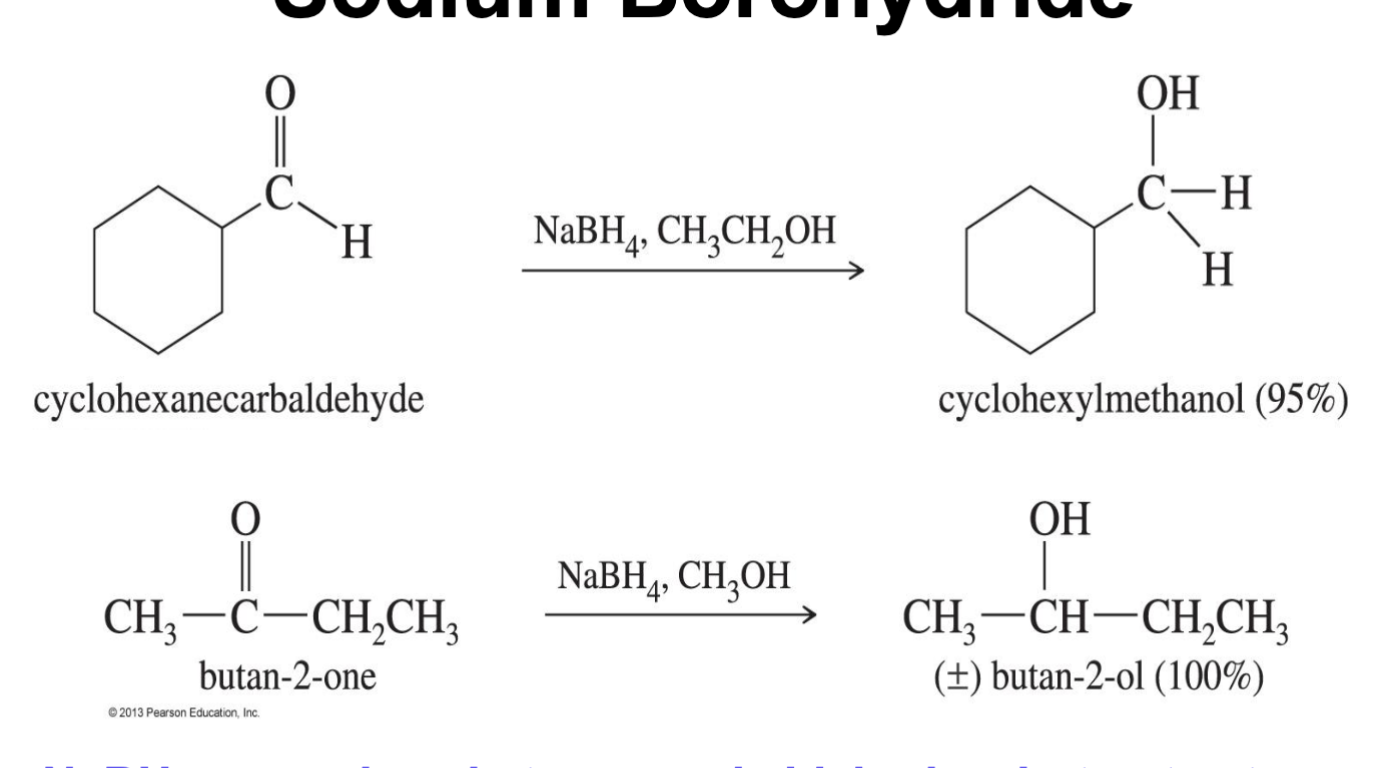
Sodium Borohydride
Reduction reagent
NaBH4
Reduces keton to secondary alcohols and aldehydes to 1alcohols
Cant reduce ester or carboxylic acid, acyl chlorides or amides
convertes the Oxygen to an alcohol
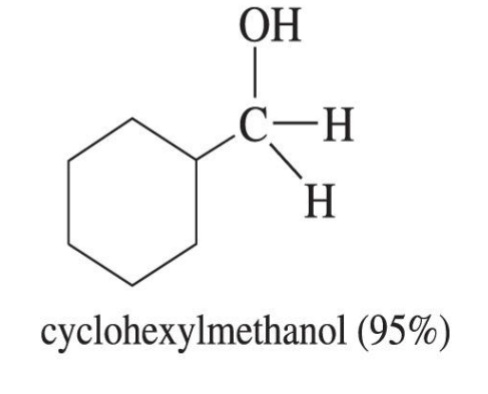
Lithium Aluminum Hydride
Reduction reagent
LiALH4
Ether
Can only reduce carbonyl (ketone and aldehyde) because it is a very strong reducing agent
Makes O → OH
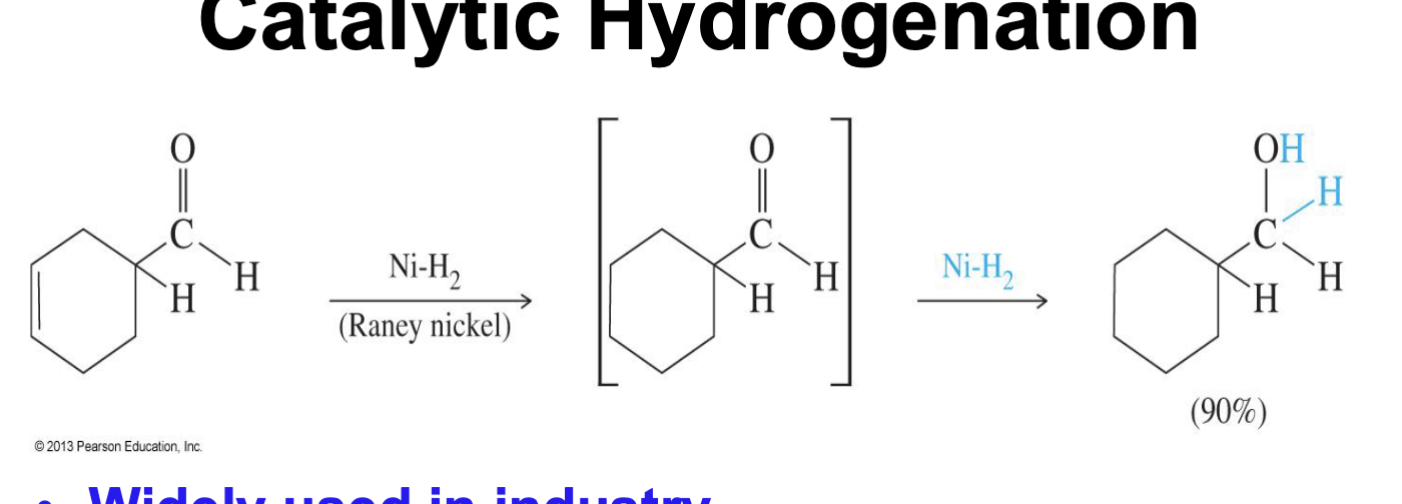
Catalytic Hydrogenation
Reducing reagent
NiH2
it will attack the alkene (C = C ) first
then it attacks the carbonyl
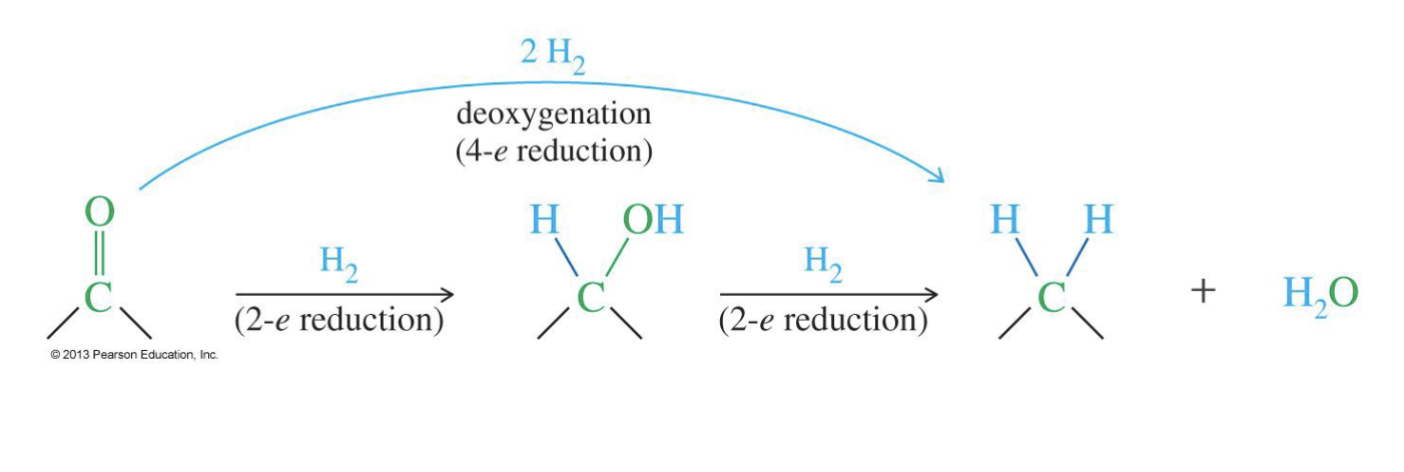
Deoxygenation of Ketones and Aldehydes
Reagent
H2
The Clemmensen reduction or the Wolff–Kishner
reduction can be used to deoxygenate ketones and aldehydes
Adds two hydrogen the same carbon so removes the double bond with Oxygen