New Material Final Exam Lecture 3/5: Cancer Genetics
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
53 Terms
what is cancer
a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body
genetic disorder involving muts in cells
not inherited but certain inherited muts can predispose a person to cancer
what do malignant tumors do
disrupts surrounding tissue
what is metastasis
cancer moves to new loactions
can muts in any gene lead to cancer
no → only genes that are integral in cell division (repairing dna damage, stopping cell cycle, etc) are typically associated w cancer but other ones can j b muts in proteins, etc
what is oncogenesis
how normal cells turn cancerous
begins w loss of cell cycle control (makes tumour)
what is metastasis
tumor cells that undergo further changes that allow them to invade and disrupt other tissues
t/f: cancer cells have a lower rate of mut than normal cells
explain
false → have higher
enzymatic systems that repair cells damage or mistakes during rep are often defective
muts in genes involved in repair = more muts
more muts = more pot muts in genes involved in repair/cell cycle control
positive feedback loop
more muts → higher rate of new muts → more muts, etc
what do mistakes in DNA replication and repair lead to as a result of uncontrolled cell division (besides tumours)
genomic instability → many cancer cells have major chrom abnormalities, including missing/extra chroms and large chrom rearrangements
will end up not being able to do their desired fx bc so many muts
does a single mut in a gene involved in cell cycle control/dna repair result in cancer
no → multiple muts in those genes are req for a cell to become cancerous
how does the chance someone gets cancer change w age
increases
supports idea cancer involves an accumulation of muts
do samples from tumors only have one active allele in X-linked genes
yes
X-inactivation → whole tumor is a result of one OG cell → if cancer occured/started in 2 diff types of cells then you could see both alleles representaed
what are cell cycle checkpoints and what do they involve
control mechanisms to ensure proper progression
monitor major events in cell cycle (eg growth, dna replication, proper chrom segregation)
involve diff CDK complexes (cyclin-dependent kinase complexes)
what are cyclins
proteins that appear at diff stages in the cell cycle → bind to CDK proteins and activate them
what do CDK complexes do
phosphorylate genes → this can help activate or deactivate them → regulates their activity
modulated by cyclins
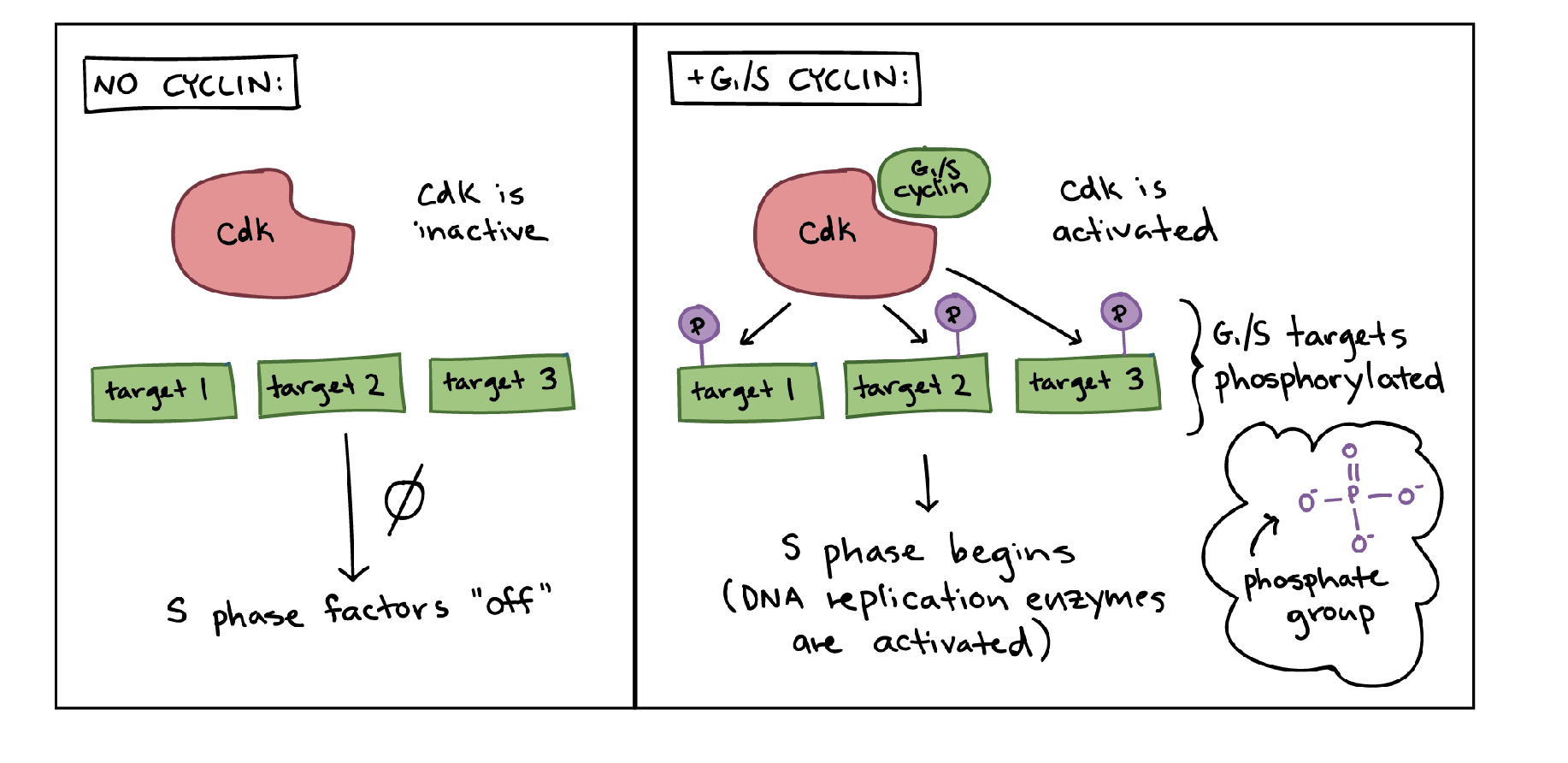
what are driver muts and what are the 2 ways they work
muts that promote cancer initiation and/or progression
inactivate tumor suppressors or activate oncogenes
have pot to inc net cell growth
eg muts in TP53, RB, BRCA
cancer generally occurs when multiple driver muts are acquired
whatare passenger muts? explain
muts that do NOT contribute to cancer
arise bc of inc muts due to driver muts
make up majority of muts found in mature cancers
what are the two classes of genes that lead to loss of cell cycle control? are they activated or inactivated by mutations and do the mutant alleles act dominantly or recessively
tumor-supressor genes (loss of fx muts)
involved in cell cycle control or repairing dna
inactivated by muts
alleles act recessively (ie need both alleles to b activated by muts to have loss of fx and inc proliferation)
oncogenes (gain of fx muts)
stimulate cell proliferation
activated by muts
mut alleles act dominantly (ie only need a mut in one wild type allele to simulate cell proliferation)
what are 2 examples of driver muts
tumour suppressor genes and oncogenes
what are RB, P53, and BRCA1/2 considered
tumour supressor genes
what is RB? what do muts in them result in
prod by a tumour suppressor gene
normal proteins that delay entry into S-phase until cell is ready to divide
muts in RB gene causes retinoblastoma (most of the time)
one or both muts can be somatic BUT way more likely to cause retino if 1 is germ
what are P53? what do muts in them result in
normal protein fxs in the G1-S checkpoint
mut gene in more than 50% of human cancers
leads to many chrom abnormalities (uncontrolled cell division)
what are BRCA1 and BRCA2? what do muts in them result in
part of machinery for repairing double-stranded breaks in DNA
muts in these genes lead to sig inc in risk for breast/ovarian cancer
without functional Rb, S-phase can not be prevented
EF2 activates transcription of S phase genes w out any repressor to pause it → uncontrolled cell growth
what is rentinoblastoma
cancer of retina
caused by muts in RB gene
some families have a dominant genetic predisposition to certain types of cancer (eg retinoblastoma)
which individuals are more prone to retinoblastoma
people who interit one copy of the RB- (dysfunctional) allele
considered a dom predisposition to cancer BUT cancer bc of tumor -repressors is a recessive trait on a cell level (2 mut alleles needed for cancer)
t/f: everyone who inherits an RB- allele gets cancer
false → only 75% penetrance
still needs other copy to mutate before they get it
note: also has varying expressivity (ie can only be in one eye)
are muts in the Rb gene recessive or dominant
recessive → 1 copy of Rb can suppress E2F on its own
what are E2F proteins? how are they regulated
a family of transcription factors that control the expression of genes needed for a cell to enter and progress through S phase of the cell cycle.
Key role:
When Rb (retinoblastoma protein) is bound to E2F → E2F is inactive, so S-phase genes stay off.
When Rb gets phosphorylated by cyclin–CDK complexes → E2F is released and activated.
t/f: an individual w RB- gene has a higher likelihood of developing retinoblastoma in both eyes than those w 2 working
true → need functional mut in both eyes individually to mutate to get retinoblastoma
person w out muts needs two muts in the SAME CELL in BOTH EYES to get it
person w RB- only needs one mut in each eye for both to have
which phase does Rb act as a gatekeeper for
S phase
it is a transcriptional repressor when unphosphorylated
how does Rb work
transcriptional repressor when unphosphorylated
Rb is usually bound to E2F (blocks promoter)
when cell is ready to go to S phase, Rb becomes phosphorylated (by cdk complex)
unbinds from E2F protein and genes required to go to S phase are transcribed
which genes control the phosphorylation of Rb
(active) CDK phosphorylates Rb
Rb no longer attaches to E2F
genes for dna rep are expressed
cell commits to S phase
t/f: tumour supressor genes usually need both alleles to be inactivated to lead to cancer
true
hereditary vs sporadic retinoblastoma
hededitary:
all cells have one mut allele
one cell aquires the second somatic mut
Sporatic
one cell aquires 2 somatic muts
diagnosed later in life
what does P53 do
protein that detects dna damage
what does protein p21 do
stops the S phase
inactivates the CDK complex
cell cycle arrest
what is TP53? explain how it works
gene that codes for p53
p53 is a tumor suppressor protein that helps maintain genome integrity by preventing cells with DNA damage from dividing.
How it works:
DNA damage or stress → p53 levels rise.
p53 can halt the cell cycle (by activating p21, which inhibits cyclin–CDK complexes) so the cell has time to repair DNA.
If damage is too severe, p53 can trigger apoptosis (programmed cell death) or senescence.
Normal role: Prevents cancer by ensuring only healthy, correctly replicated DNA is passed on.
Cancer link: Mutations in TP53 are found in more than half of all human cancers. When p53 is lost or defective, cells can divide unchecked even with severe DNA damage.
If E2F and cyclins are the “go” signals for division, p53 is the emergency brake—and in cancer, that brake often fails.
tumour suppressor gene
transcription factor (activator NOT repressor → activates P21 gene - inactivates CDK complex)
constitutively expressed but is rapidly degraded
doesn’t really work unless stabilized
phosphorylation of p53 stabilizes it → it becomes an active transcription factor
p21 inactivates CDK complex
RB gate stays closed = cell cycle arrest
if damage cannot be repaired, p53 initiates apoptosis
p53 acts as brake for progression of the cell cycle
what phosphorylates p53? why does that phosphorylate it
cell damage/stress
activates p53 → activates p21 → inhibits cdk complex → cell cycle arrest bc its damaged → gives time to repair OR if enough phos p53 accumulates, apoptosis
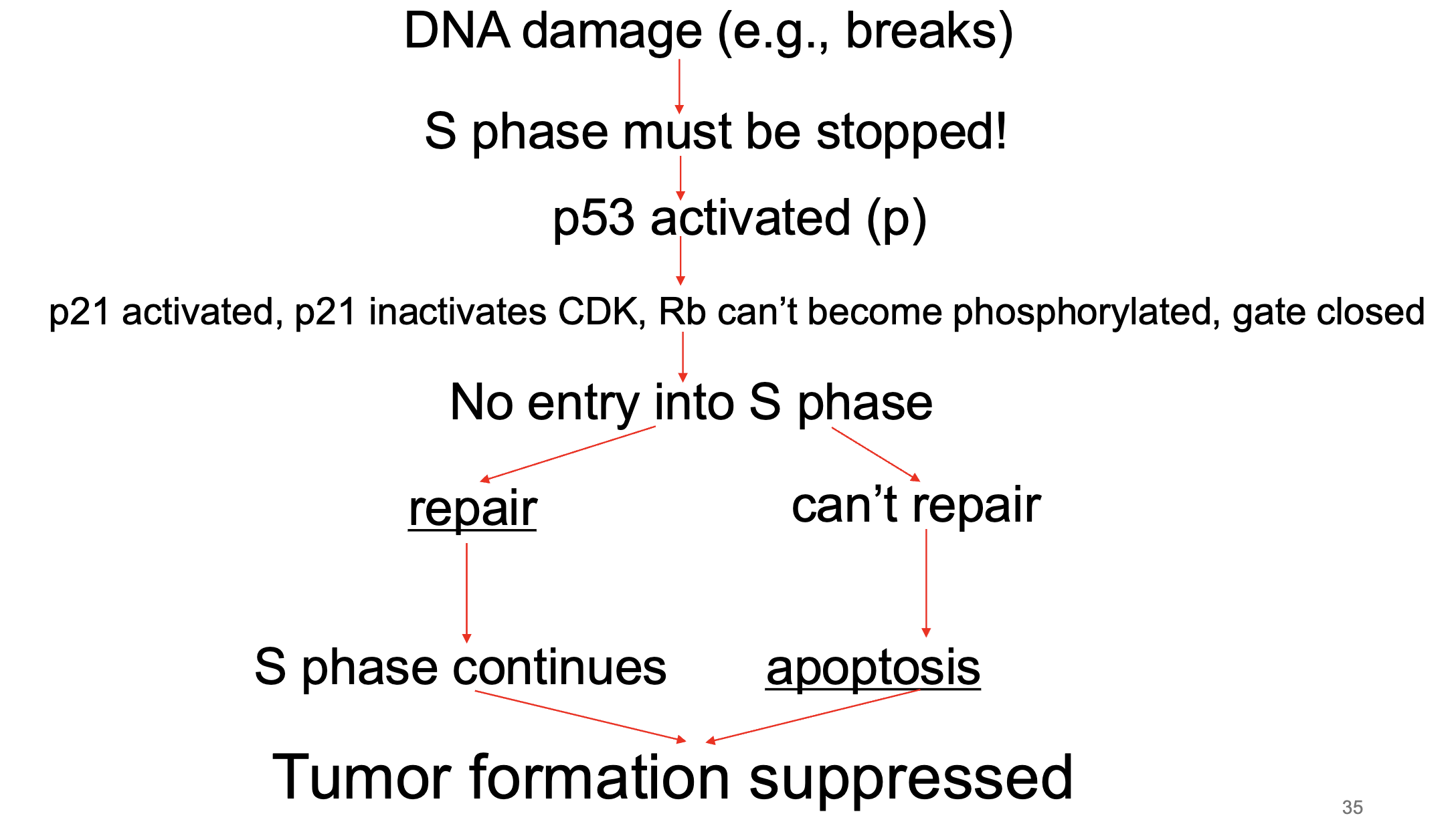
what happens w out functional Rb and p53
rapidly rep cells eventually acquire dna damage (naturally or mutagens)
nothing to stop cell cycle to fix damage → muts occur and tumorigenesis (also called oncogenesis or carcinogenesis) begins
loss of cell cycle control inc rate of new muts
leads to cancers if enough muts accumulate
why don’t muts in a single gene involved in the cell cycle generally cause cancer
bc cell cycle has multiple diff checkpoints (backup systems)
why are we concerned with p53 muts and uncontrolled cell division if telomeres will eventually run out and the cells will die anyways
inc chance muts controlling telomerase
expression allow telomeres to be lengthened → cells basically become immortal
muts like TP53 inc likelihood for muts in general, including other ket genes like telomerase gene
do the BRCA1 and BRCA2 genes have high or low penetrence
high penetrence → 90% for BCRA1 mutant alleles and 41% for BRCA2
what are BRCA1 and BRCA 2? what do muts in these result in
tumour suppressor genes
gene products are parts of a surveillance system needed for repairing dna breaks
when mutated, damaged dna may get replicated
heterozygotes are at higher risk for acquiring muts in remaining copy compared to individuals not born w mut
promotes muts in other genes leading to cancer = phenotypic dominance
are mutant BRCA1 and 2 phenotypically dominant or recessive for cancer
recessive → when mutated, damaged dna may get replicated
heterozygotes are at higher risk for acquiring muts in remaining copy compared to individuals not born w mut
promotes muts in other genes leading to cancer = phenotypic dominance
what are proto-oncogenes
normal genes that mutate to form oncogenes
a proto-oncogene is a gene w normal fx in a cell (usually related to cell growth/differentiation)
encode proteins needed for cell cycle progression
gain of fx muts result in increased proliferation
gain of fx muts can arise from insertion of a strong viral promoter adjicent to the proto-oncogene
what are oncogenes
mutated forms of proto-oncogenes → causes cell to reprod rapidly
excessive proliferation inc chances of more muts which could lead to cancer
what is the gene that encodes for the ras protein an example of? explain why
a proto-oncogene
codes for a family of proteins that act as molecular switches in cell growth and differentiation
in normal cells, ras is activated by binding of growth factors to the cell
activation of ras leads to cell proliferation
when ras genes are mutated (eg in cancer cells) ras is active even in absence of growth factors
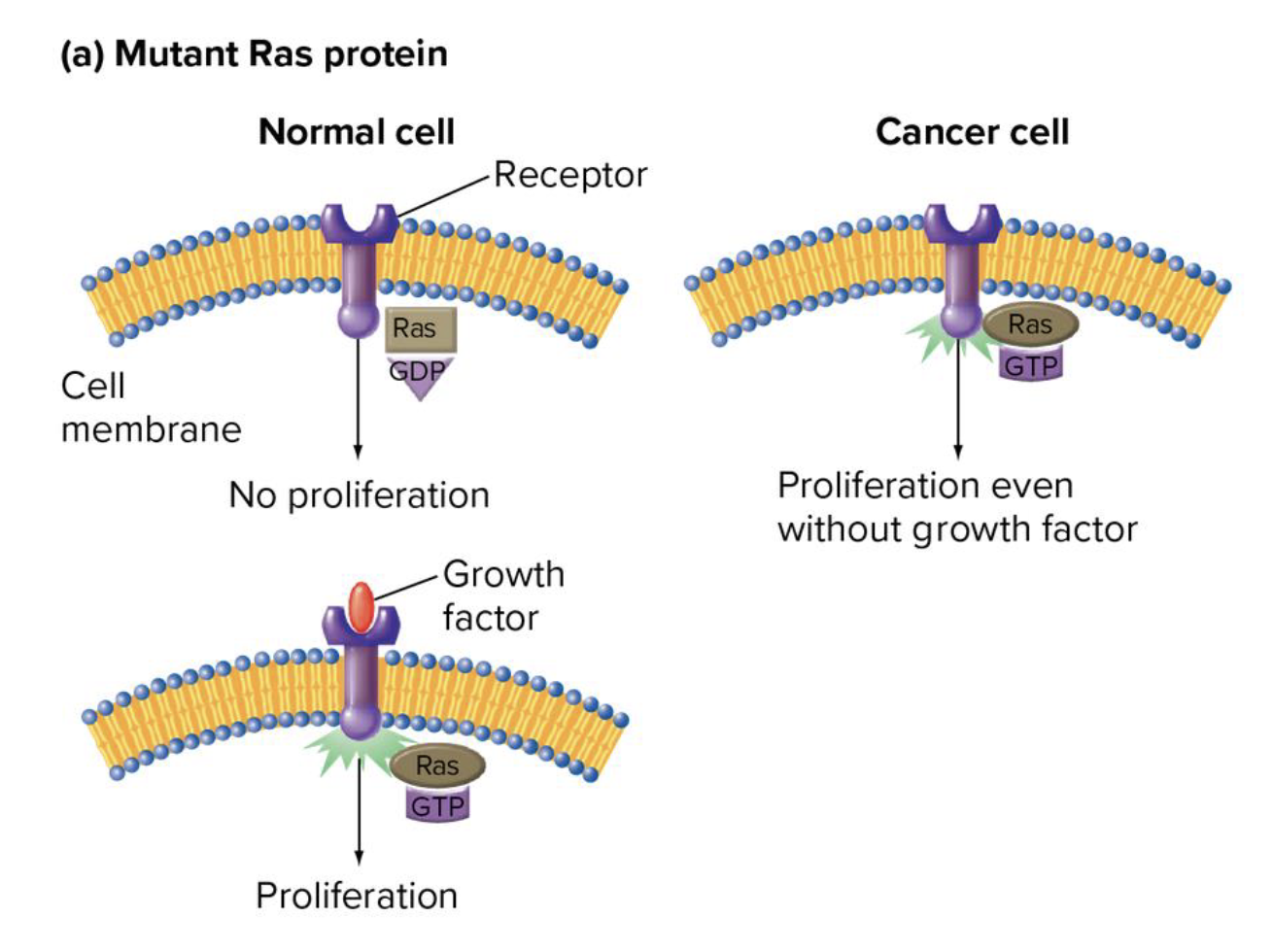
are muts in ras gain or loss of fx muts
gain of fx muts
what studies helped lead to the discovery of specific proto-oncogenes? how
studying tumour-causing reteroviruses (oncoviruses)
some viruses inc rate of cancer
looking at genome of tumour-causing virus can reveal oncogenes
looking at genomes of tumours induced by these viruses can also reveal oncogenes
viral dna is relatively easy to find → identifying where in host genome it has integrated to cause tumours can help identify proto-oncogenes
are cancer causing viruses lytic dna viruses
no → do not promote cell lysis
instead they’re reteroviruses
what are reteroviruses? what do they do
RNA viruses
most cancer causing viruses
use an enzyme called reverse transcriptase → converts their RN genome into DNA
that viral dna integrates into host genome
BUT they do not become viral particles and exit the genome and cell like lytic ones do
once integrated into host genome, the viral genes are often transcriptionally active → strong/constitutive viral promoters
can be located anywhere in genome → can be located upstream of proto oncogenes (now making them onco)
oncogene gets transcribed along w viral genome and gets packaged into progeny virus particles which insert them into other hosts/cells
this dna is generally permanently inserted into the host genome
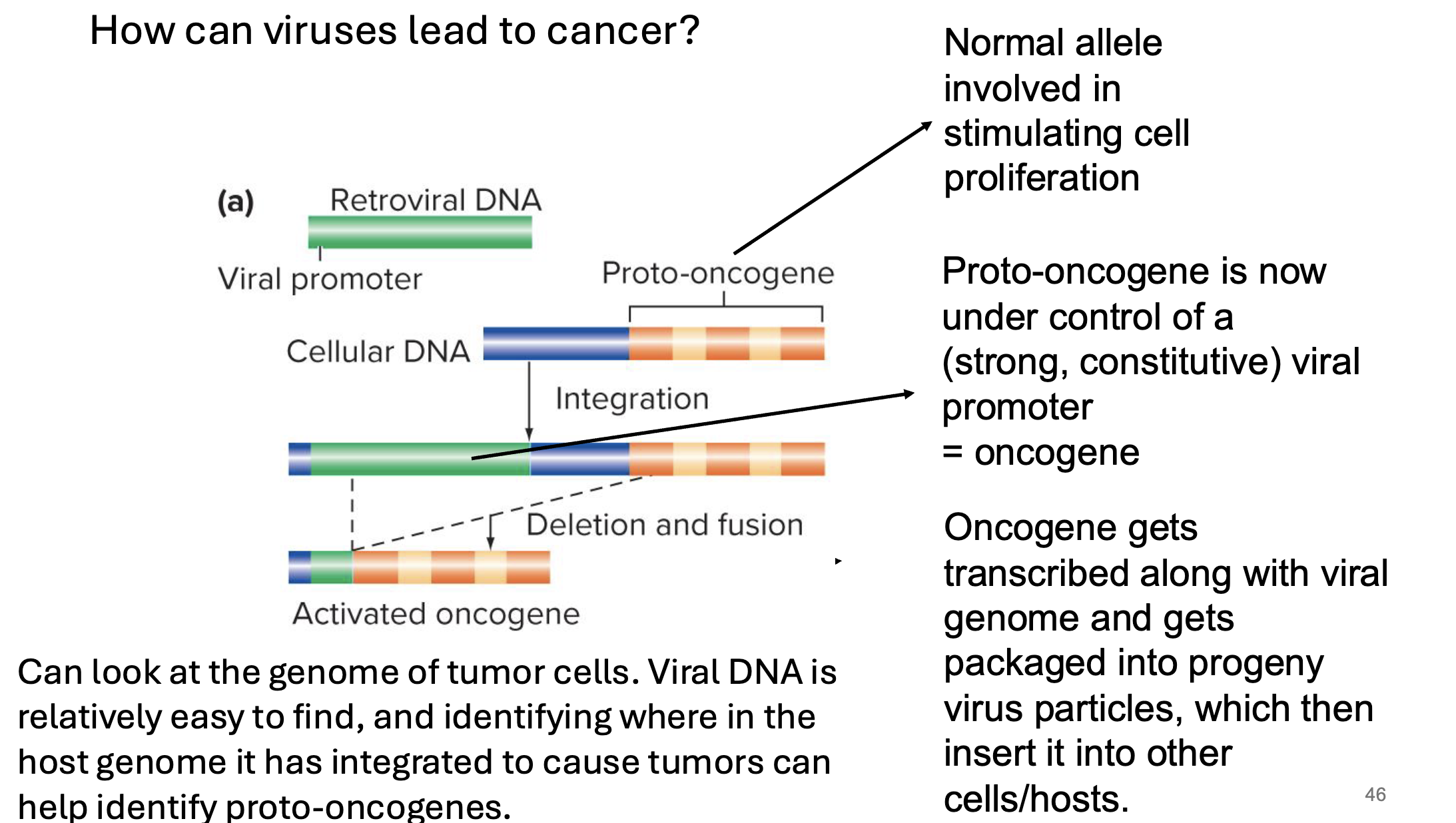
are ongogenes dominant of recessive for cancer
dominant