Chemistry Unit 1 AOS 2
1/96
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
97 Terms
Define relative isotopic mass:
Mass of a single atom of a particular isotope, relative to the mass of a single carbon-12 atom
Define relative abundance:
Percentage of a particular isotope found in a natural sample of an element
Define relative atomic mass (Ar):
Weighted average of all the individual relative isotopic masses of an elements
How to calculate relative atomic mass?
Total relative abundance of isotopes of an element must always add up to ____%
100%
Solve this:
If the relative atomic mass is 79.9 and the isotopes are 79Br and 81Br, what is the relative abundance?
Define relative molecular mass (Mr):
The total mass of a molecule, based on all its atoms, measured on a scale where carbon-12 is exactly 12/ sum of the relative atomic masses of each atom in a molecule
How to calculate molecular mass?
Calculating the molecular mass = number of atoms in each element x relative atomic mass of each element
Calculate the molar mass of H20:
Define relative formula mass and how to calculate it?
The total mass of atoms in an ionic compound's formula unit, measured on a scale where carbon-12 is exactly 12.
Ionic compounds form continuous crystal lattices
Calculated in the same way as molecular mass
What is mass spectrometry? What instrument does it use?
Technique used to measure the relative abundance of different isotopes of an element
Uses a mass spectrometer
Outline the process of mass spectrometry:
The sample of the element is turned into cations by removing electrons
The ions move through the machine
--> How they move depends on their mass-to–charge ratio (m/z)
The machine creates a graph called a mass spectrum
--> In the graph, each peak represents an isotope
In a mass spectrum, what does the height (y-axis) and the horizontal axis (x-axis) represent?
The height (y-axis) indicates how abundant the isotope is as a percentage of the total mass of the same/relative isotope
The horizontal axis (x-axis) shows the mass-to-charge ratio (m/z) which is equal to the relative isotopic mass of each isotope (as each isotope of an element has the same charge)
In a mass spectrum, all the peaks added up together are equal to a total of ____ of _____.
100%, relative abundance
Calculate the relative abundance of each isotope/peak:
What is the Avogardo’s constant/number (NA)?
Refers to the number of particles in one mole of a substance
It is equal to 6.02 x 1023
What do these symbolise?
n
NA
N
Number of moles
6.02 x 1023 / Avogardo’s constant
Total number of particles
How to calculate each of these using number of moles (n), avogardo’s constant (NA) and number of particles (N)?
n
N
NA
n = N/NA
N = n x NA
NA = N/n
In H20, how many hydrogen and oxygen moles are there in one mole of water molecules?
Hydrogen: 2 moles of atoms
Oxygen: 1 mole of atoms
Define molar mass (M):
Is the mass, in grams, one of one mole of an atom or molecule
True or false?
Relative molecular masses and molar masses are exactly the same → This includes both value and unit used.
No
While the values are the same, the unit used are not the same
Relative molecular masses do not use units whereas molar masses use g mol-1
What do these symbolise?
m
n
M
Mass (grams)
Number of moles
Molar mass
How to calculate each of these using mass (m), n (number of moles) and M (molar mass?
m
n
M
m = M x n
n = m/M
M = m/n
Define percentage composition and how to calculate it?
Percentage of mass of an element in a compound
Find the percentage composition of carbon in CH4:
Define empirical formula:
Chemical formula depicting the lowest whole number ratio of atoms of different elements in a compound
How to determine empirical formulas?
Mass of each element (m)
Number of moles (n)
Divide all elements' n by the smallest n out of all elements (n/smallest n)
Express as an empirical formula
Find the empirical formula of a compound with nitrogen and oxygen? If we know that 30.4% of the compound’s mass is Nitrogen?
Solve this:
Define molecular formula:
Actual number of atoms of each element in a molecule
How to calculate the molecular formula?
Empirical formula x molar mass of compound/molar mass of empirical formula
If the empirical formula of a compound is CH2O and the molar mass of the compound is 60.0 g mol-1, then determine the molecular formula of the compound
What are hydrocarbons?
Organic compounds consisting of carbon and hydrogen atoms
What are organic compounds?
Compounds are carbon-based, and can either be naturally and synthetically produced
Define homologous series:
Family of compounds that:
Similar physical and chemical properties
Similar structure
Same general formula
Differ from the number of carbon atoms in the chain
What are the common elements that bond with carbon?
H,O,N,S and Cl
hydrogen, oxygen, nitrogen, sulfur, and chlorine
What are the two main classes of hydrocarbons? Compare both (mention their general formulas, functional groups and saturation/unsaturation)
Alkanes
Homologous series of hydrocarbons made up of only single covalent bonds
CnH2n+2 (N = number of carbons in parent chain)
Saturated (Hydrocarbon possessing only single bonds between carbon atoms + each carbon cannot bond with any more atoms)
Alkenes
Homologous series of hydrocarbons that contain a double bond/s between carbon atoms
Carbon to carbon double bond functional group
CnH2n
Unsaturated (Hydrocarbon possessing at least one double bond between carbon atoms + carbon atoms can form new bonds with other atoms)
Both alkanes and alkenes are ____ molecules and hence are ____ in water
Non-polar, insoluble
Both alkanes and alkenes have ____ forces, however as the size of the molecule ___, the stronger the dispersion forces
Weak dispersion, increases
Stronger forces result in ____ boiling and melting points, as well as ___ volatile (ability to turn into gas) and ____ viscosity (thickness)
Higher, less, higher
Compare the physical and chemical properties of alkanes and alkenes:
Physical
Alkanes and alkenes have similar physical properties
--> Low boiling points (compared to polar molecules) due to weak intermolecular forces
Chemical
Alkanes are relatively unreactive + but undergo substitution reactions (a hydrogen atom gets replaced by another atom)
e.g. CH₄ + Cl₂ → CH₃Cl + HCl
(Methane + Chlorine → Chloromethane + Hydrogen chloride)
All alkanes can undergo combustion reactions - react with oxygen to produce energy, carbon dioxide and water--> so they are used as fuels
--> This an exothermic reaction (releases energy in the form of heat + light)
Alkanes can also undergo substitution reactions in the presence of UV light --> where a hydrogen atom in their structure gets replaced by a halogen
Whereas
Alkenes are more reactive + undergo addition reactions (double bond opens up and new atoms gets added)
e.g. C₂H₄ + Br₂ → C₂H₄Br₂
(Ethene + Bromine → Dibromoethane)
--> useful in chemical manufacturing, as they can easily be turned into different compounds
What determines the parent name?
The number of carbon atoms in the parent chain/longest unbranched chain of carbons
What do the green and blue parts indicate:
Green: Indicates number of carbons in parent chain
Blue: Type of bonding between the carbons in the carbon chain
What suffix is used for alkanes?
'-ane'
What suffix is used for alkenes?
-ene
Why doesn’t methene exist?
As double bonds between carbon atoms cannot occur with only one carbon atom present
Fill out this table:
Carbon chain length | Parent name | Example Alkane | Example Alkene |
1 | x | ||
2 | |||
3 | |||
4 | |||
5 | |||
6 | |||
7 | |||
8 |
Carbon chain length | Parent name | Example Alkane | Example Alkene |
1 | Meth | Methane | x |
2 | Eth | Ethane | Ethene |
3 | Prop | Propane | Propene |
4 | But | Butane | Butene |
5 | Pent | Pentane | Pentene |
6 | Hex | Hexane | Hexene |
7 | Hept | Heptane | Heptene |
8 | Oct | Octane | Octene |
What’s the name of this molecule?
What are structural isomers?
Isomers that have the same molecular formula but the atoms are arranged in a different spatial arrangement/different structural formula
What are alkyl groups? What suffix do they use?
Side-chain attached to parent carbon chain, containing only carbon and hydrogen atoms
Uses suffix ‘yl’
What’s the name of these molecules?
2-methylpropane
When compounds have more than one side group bonded to parent chain --> alkyl groups are named in ____ order
Alphabetical
What is the name of this compound:
4-ethyl-2-methyloctane ('e' goes before 'm')
What is the name of this compound and the other structural isomers?
The semi-structural formula has ____ when a alkyl group is present. What is the semi-structural formula of this:
Brackets
CH3CH(CH3)CH2CH3
Define functional groups:
Specific group of atoms or atom within a molecule that determine the properties of a molecule
Define haloalkanes: State its general formula and the elements that can be X:
Organic compounds that contain a halo functional group (-X) which replaces a H atom on an alkane
General formula: CnH2n+1X
Halogen atom : F, Cl, Br, I
Comment on the properties of haloalkanes:
Halogens are highly reactive (group 17) --> the difference in reactivity between halogen atoms and carbon atoms = polar covalent bond and hence form permanent dipole-dipole bonds
--> As a result, haloalkanes have a higher boiling point than other alkanes or alkenes (that have weak dispersion forces)
However as the haloalkane molecule increases --> the overall molecule becomes nonpolar as the C-X bond becomes less dominant --> intermolecular forces weaken
Haloalkanes are slightly soluble as they can form dipole-dipole interactions with water molecules (polar C-X bond) + but the rest of the molecule is nonpolar and the C-X bond is strong
Complete this table:
Halogen | Prefix |
Fluorine | |
Chlorine | |
Bromine | |
Iodine |
Halogen | Prefix |
Fluorine | Fluro- |
Chlorine | Chloro |
Bromine | Bromo |
Iodine | Iodo |

Name these:
Define alcohol: State its general formula
Organic compound that has a hydroxyl (–OH) group which replaces a H atom on an alkane
General formula: CnH2n+1OH
Comment on the properties of alcohol:
Large difference between electronegativity between oxygen and hydrogen --> polar covalent bond
A hydroxyl group can take part in permanent dipole-dipole interactions and hydrogen bonding --> strong molecular forces, hence higher boiling point than alkanes (weak dispersion forces)
However as the alcohol molecule increases --> the overall molecule becomes more nonpolar as the hydroxyl group becomes less dominant --> intermolecular forces weaken
--> this causes the bp to decrease
--> solubility of the molecule in water to also decrease (as it becomes less polar)
How to name alcohol?
When naming alcohol, the 'e' at the end is removed and replaced with 'ol'
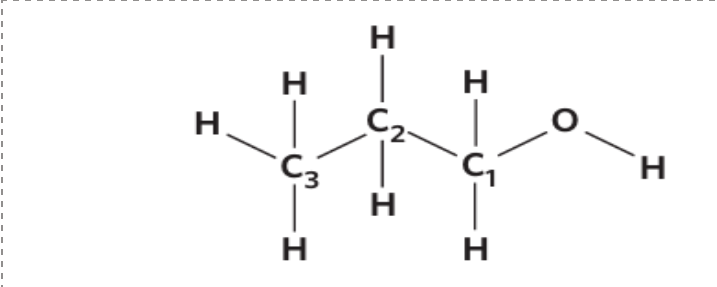
State the name of this:
Define carboxylic acids: State its general formula
Organic compound that contains a carboxyl functional group (-COOH)
General formula: CnH2nO2
Comment on the properties of carboxylic acids:
Both C=O bond + O-H bond are polar --> hence carboxylic acids form strong dipole-dipole interactions and hydrogen bonds --> high bp (contains both carbonyl group and hydroxyl group) (even higher than alcohol, haloalkanes and alkanes)
However as the carboxylic acid molecule increases --> the overall molecule becomes more nonpolar as the carboxyl group becomes less dominant --> intermolecular forces weaken
-> causing the water solubility to decrease
Weak organic acids
Found in food
Sour taste
Ionisation in water
How to name carboxylic acids?
When naming carboxylic acids, the 'e' at the end is removed and replaced with '-oic acid'
Carbon atom with carboxylic acid bonded to it --> always considered as the first carbon atom on chain
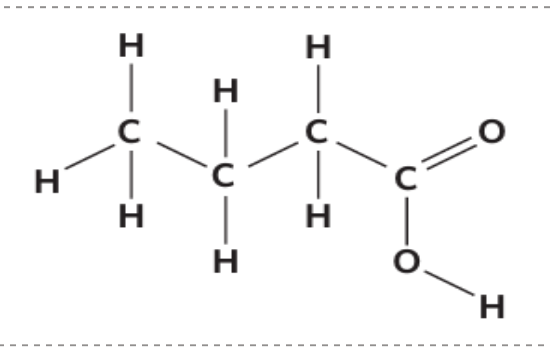
State the name of this:
Crude oil is made up of many different ____, which can be separated by fractional distillation --> this separates crude oil into fractions (groups of hydrocarbons with similar boiling points) known as fossil fuels
Hydrocarbons
List some examples of fossil fuels:
Petrol (octanes), natural gas (methane), Diesel
Plant-based biomass are organic material from plants that can be used as _____ sources of energy
Renewable
Bioethanol --> ethanol produced from the fermentation of plant sugars
Glucose (sugar) → Ethanol + Carbon dioxide
C₆H₁₂O₆ → 2 C₂H₅OH + 2 CO₂
Ethanol + Oxygen → Carbon dioxide + Water + Energy
2C2H5OH + 6O2 →4CO2 +6H2O
Photosynthesis:
6CO2 +6H2 --> C6H12O6 +6O2
What are polymers?
Covalent molecular substances made up of many repeating units called monomers
What are the different ways polymers can be produced?
Addition polymerisation
Condensation polymerisation
Compare addition polymerisation and condensation polymerisation:
Addition polymerisation | Condensation polymerisation |
|
|
What are some general qualities of polymers?
Light and strong
Durable (long-lasting/tough)
Highly versatile + modifiable
Flammable
Chemically resistant
Effective thermal + electrical insulators
Polar polymers are ____ + more ____ than non-polar polymers due to having ___ intermolecular forces (e.g. dipole-dipole, hydrogen)
Harder, rigid, stronger
How is LDPE made? And what are some of its qualities?
Polyethene/polyethylene is made by addition polymerisation of monomer ethene (C₂H₄)
Low-Density Polyethene (LDPE) : When polymerisation takes place at high temperatures + pressures, prevents the formation of linear chains and instead the chains get many branches
The branches prevent the polyethene from packing together closely --> resulting in weaker forces
As a result LDPE has low density, soft and flexible, low mp, insulator of electricity, good chemical resistance, opaque (transparent in thin forms)
How is HDPE made? And what are some of its qualities?
High-Density Polyethene (HDPE) : When polymerisation takes place at low temperatures + pressures --> less branching + polymer chains pack together tightly
As a result, HDPE has higher density, hard, high mp, insulator of electricity, weatherproof + cold resistant, allows light to pass through
What are the three major categories polymers can be separated into?
Linear (thermoplastics)
Elastomers
Cross-linked (thermosetting) polymers
Fill out this table:
Talk about how it's made
What’s it used in
Type of fossil fuel-based plastic |
|
HDPE & LDPE High-density polyethene (HDPE) and low-density polyethene (LDPE) | |
PVC Polyvinyl chloride/Vinyl | |
PP Polypropene | |
PS Polystyrene
|
Type of fossil fuel-based plastic |
|
HDPE & LDPE High-density polyethene (HDPE) and low-density polyethene (LDPE) |
HDPE
LDPE
|
PVC Polyvinyl chloride/Vinyl |
|
PP Polypropene |
|
PS Polystyrene
|
|
What are bioplastics?
Plastics produced from plant-based biomass
Fill out this table:
How its made
Type of bioplastics |
|
Polylactic acid (PLA) | |
Bio-polyethene (Bio-PE) | |
Bio-polypropene (Bio-PP) |
Type of bioplastics |
|
Polylactic acid (PLA) |
Used in:
|
Bio-polyethene (Bio-PE) |
|
Bio-polypropene (Bio-PP) |
Plant material → Methylpropan-1-ol → Dehydration → Propene → Polymerisation → Bio-PP |
Outline the process of recycling. What are the different types of recycling methods?
Sorting, washing and drying
Plastic recycling
Mechanical recycling
Chemical recycling: Changes to chemical structure to turn back into raw materials
Organic recycling/composting
What are compostable plastics? List some examples.
Biodegradable plastics --> break down quickly + do not leave toxic residue or microplastics --> instead turn into CO2 + water vapour + humus
e.g. PLA, PHB, PBAT
How can plastics contribute to the circular economy?
Using renewable plastics and changing recycling practices (chemically recycling + mechanical recycling)
Can you find the polymer of this:
Can you solve this condensation polymerisation reaction:
Can you solve this condensation polymerisation reaction:
Compare thermosets and thermoplastics in terms of recycling:
Thermosets --> cannot be recycled easily due to their rigidity and inability to melt --> hence most end up as waste
Thermoplastics --> can be recycled --> however are recycled to lower quality
What is chemical/advanced/feedstock recycling? Outline the different types of chemical recycling:
Conversion of plastics back into their monomers or organic chemicals
Plastic dissolution: dissolved in solvents using heat --> solution of polymers + additives --> separated
Chemolysis/depolymerisation: using chemical reactions to break condensation polymerisation plastics into monomers
Pyrolysis: high heating plastics without oxygen --> breaks them down into organic chemicals --> fuel
Outline the process of organic recycling:
Shredded into smaller pieces
Often reacted with hot water to break polymer chains
Microorganisms eat monomers
Release CO2, water vapour and humus
Can you solve this condensation polymerisation question:
What is hydrolysis?
Chemical reaction where a polymer is broken down into monomers by adding water
When the h20 is added --> OH goes to one piece and H to another
Opposite to condensation polymerisation
Allows for reuse of monomers/biodegradable --> can contribute to circular economy