CHEM All Lesson Prelim G12 Part 2
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
61 Terms
vocab

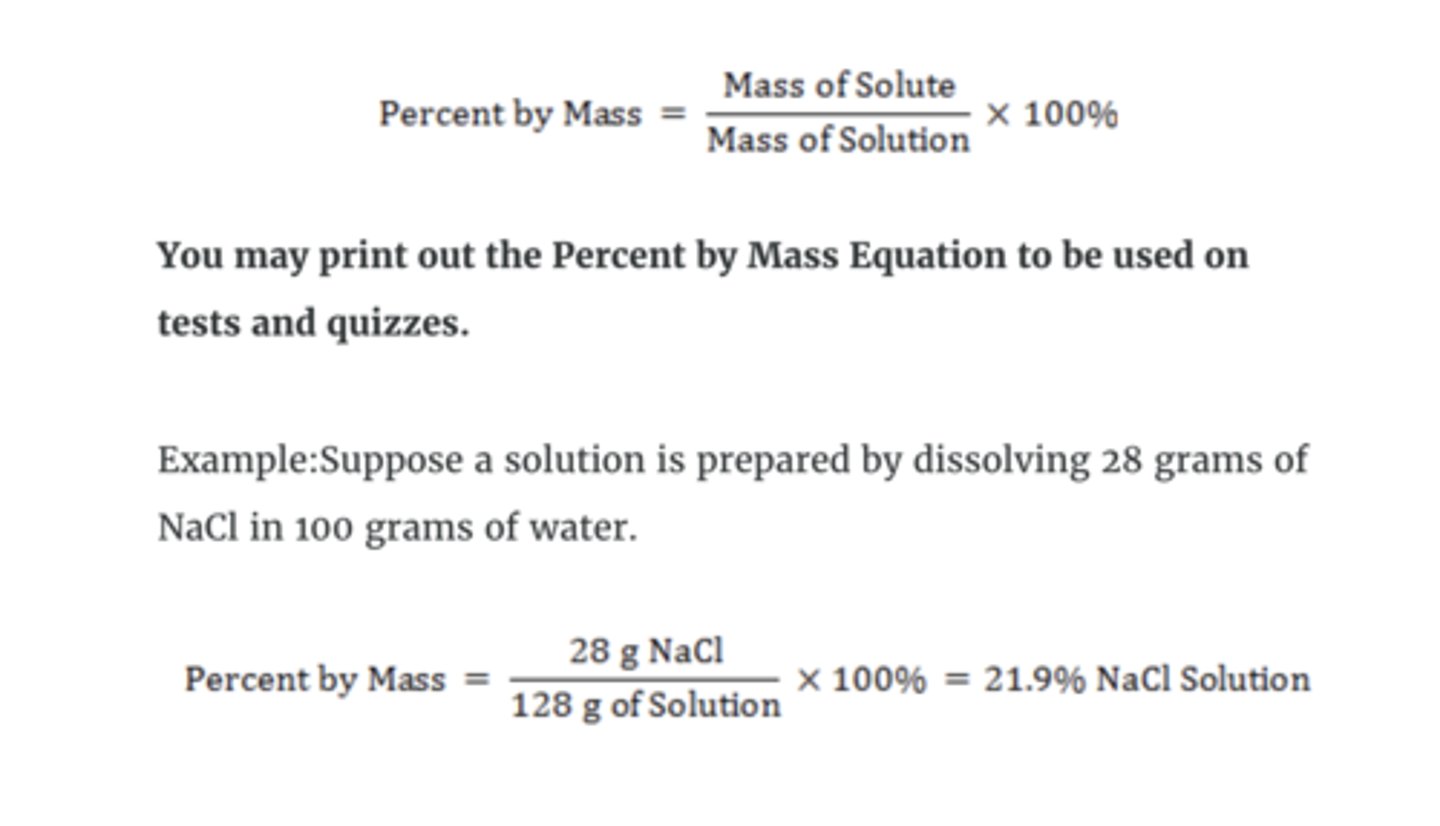
percent by mass
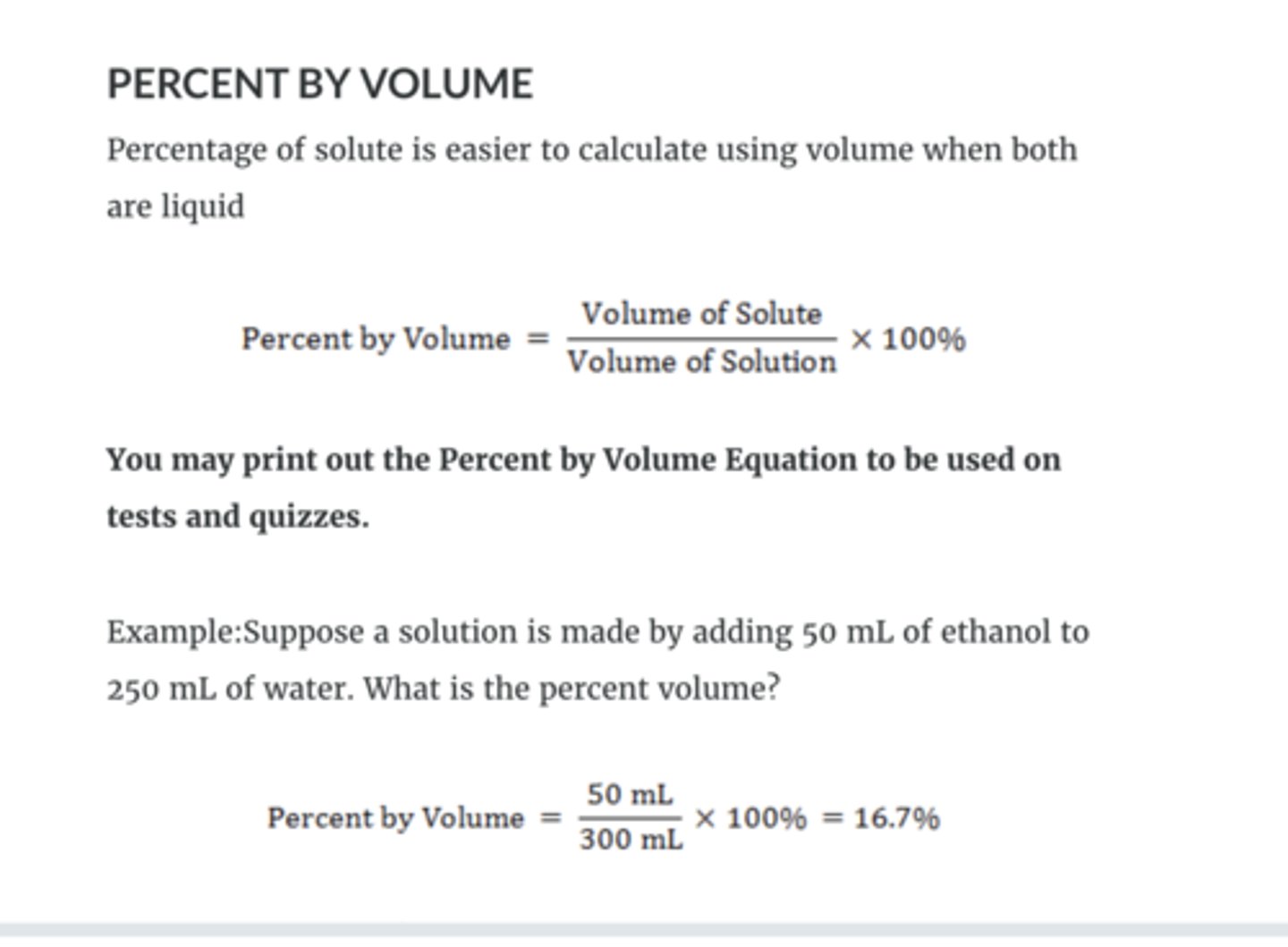
percent by volume
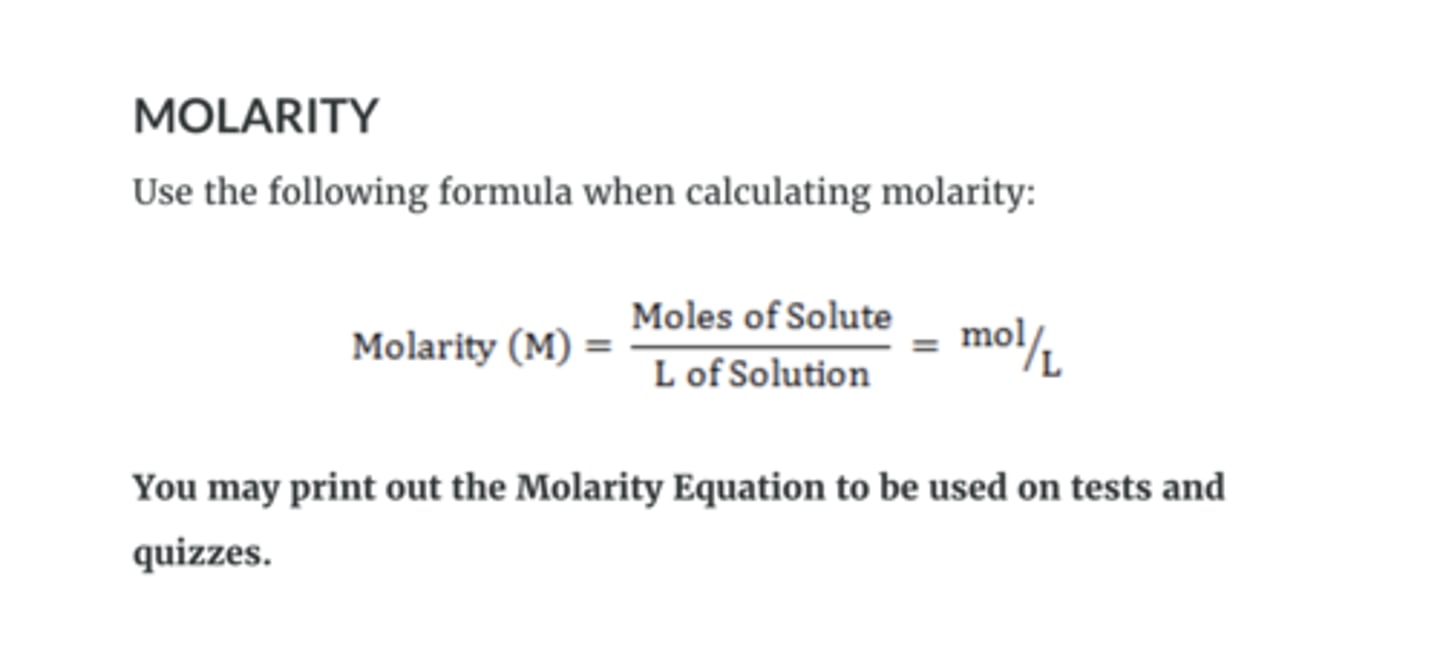
molarity
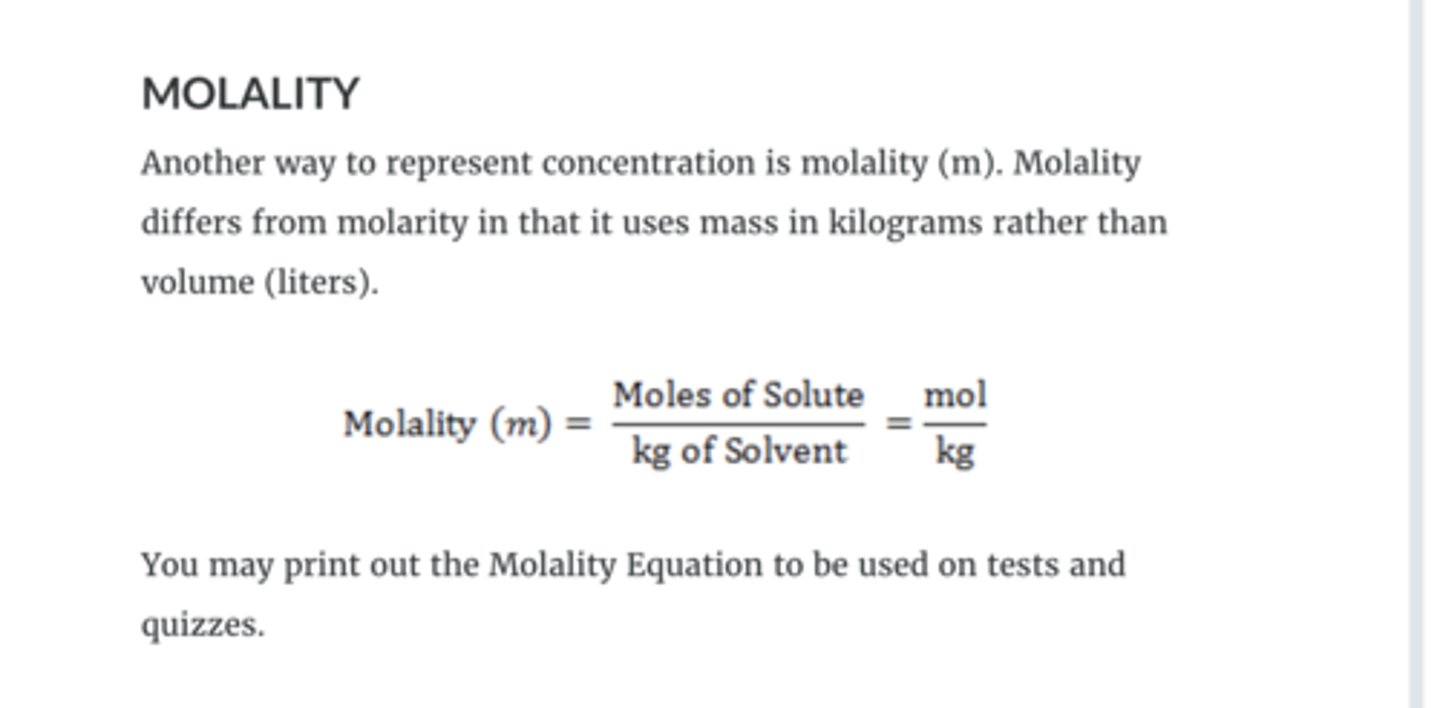
molarity2
96.0 g of glucose in 704 g of water
If you need to make 800. g of a 12.0 % by mass glucose solution, what mass of glucose needs to dissolved into what mass of water?
50.0 mL
What volume of a 40.0 % by volume acetone solution can be made with 20.0 mL of acetone?
dilute; concentrated
A solution with a relatively small amount of solute is _____, while one with a larger amount of solute is _____.
5.00 %
What is the percent by volume of a solution prepared by adding water to 30.0 mL of isopropyl alcohol until the volume if the solution is 600 mL?
moles of solute per liter of solution
What is molarity?
1.14 m
What is the molality of a solution prepared by dissolving 317 g of CaCl2 into 2.50 kg of water?
moles of solute per kilogram of solvent
What is molality?
40.0 g
To make 1.00 L of 1.00 M NaOH solution, what mass of NaOH needs to be weighed out?
0.2m
What is the molality of a solution prepared by dissolving 0.40 mol into 2.0 kg of solvent?
0.125 mol
How many moles of Ca(NO3)2 are there in 500 mL of 0.25 M solution?.
0.50 M
2.0 L of a 1.5 M solution is diluted with water until the final volume is 6.0 L. The molarity of the solution is now _____.
0.282 M
0.550 moles of NaCl are dissolved in 1.95 L of water. Calculate the molarity of the NaCl solution.
It is cut in half.
How does doubling the volume of a solution by adding solvent affect the molarity?
250 mL
The most highly concentrated hydrochloric acid is 12 M. What volume of a 12 M stock solution of HCl should be diluted in order to produce 6.0 L of 0.50 M HCl?
6.25%
What is the percent by mass of a solution made by dissolving 10.0 g of NaCl into 150.0 g of water?
.34 L
45.0 g of Ca(NO3)2 was used to create a 0.80 M solution. What is the volume of the solution?
Solids
A state of matter that has a definite shape and definite volume. Its particles are tightly packed, making it non-compressible. The particles only vibrate in place and are not free to move. They are held together by strong forces such as ionic or covalent bonds.

Crystalline Solids
A type of solid characterized by an ordered structure with a regular repeating pattern. They have well-defined melting points and highly regular shapes with relatively sharp edges. These solids are anisotropic, meaning their physical properties vary depending on the direction in which they are measured. Held together by various types of bonds, including covalent bonds, ionic bonds, Van der Waals forces, and metallic bonds.

Amorphous Solids
A type of solid that lacks an ordered internal structure, making them shapeless. They do not have well-defined melting points and break into fragments with irregular shapes. They are isotropic, meaning their physical properties are the same in all directions. They typically consist of covalently bonded networks.

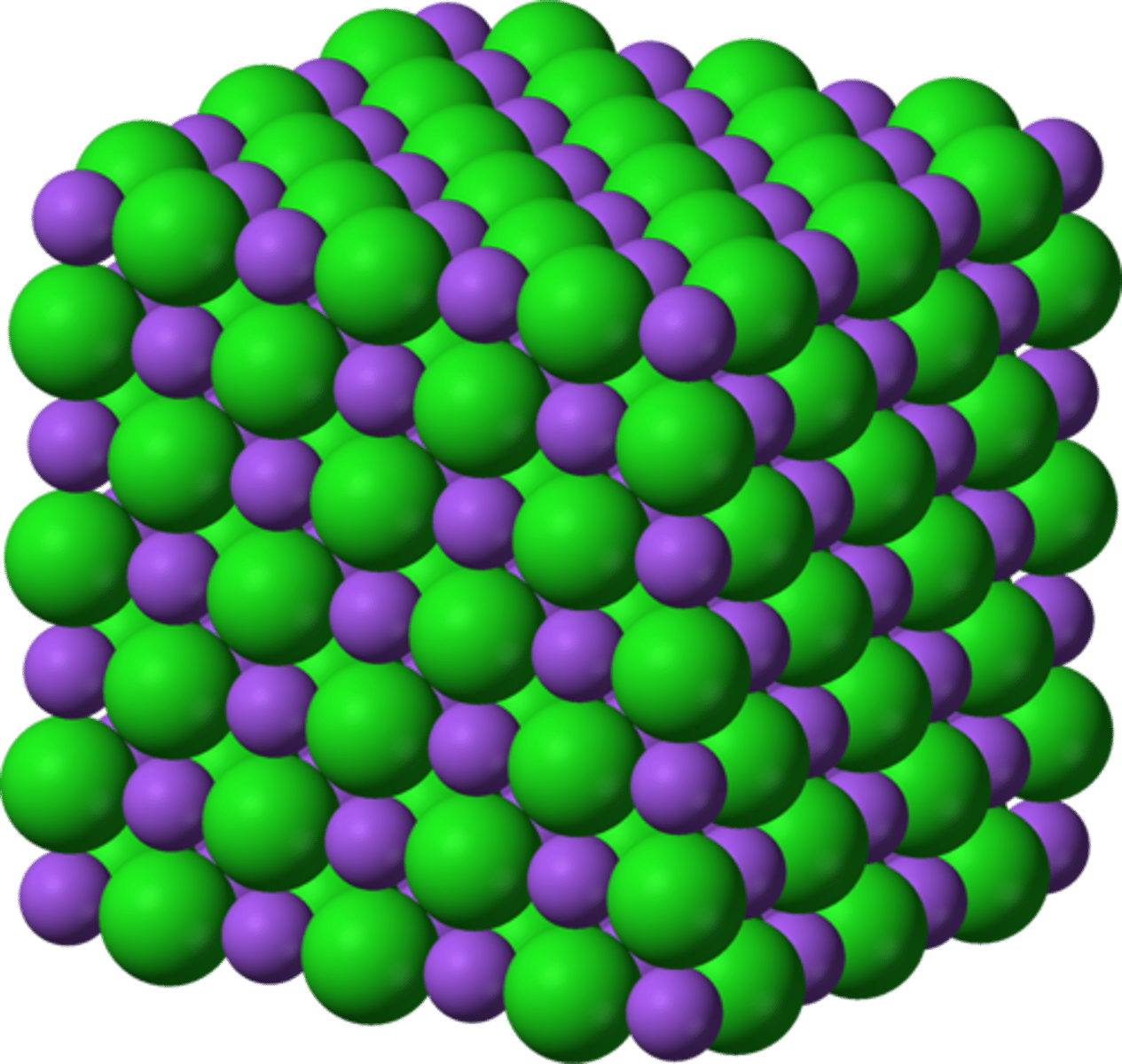
Ionic Solids
Composed of positive and negative ions held together by strong electrostatic attractions. They are characterized by very high melting points, brittleness, and poor electrical conductivity in the solid state. A common example is NaCl (table salt).
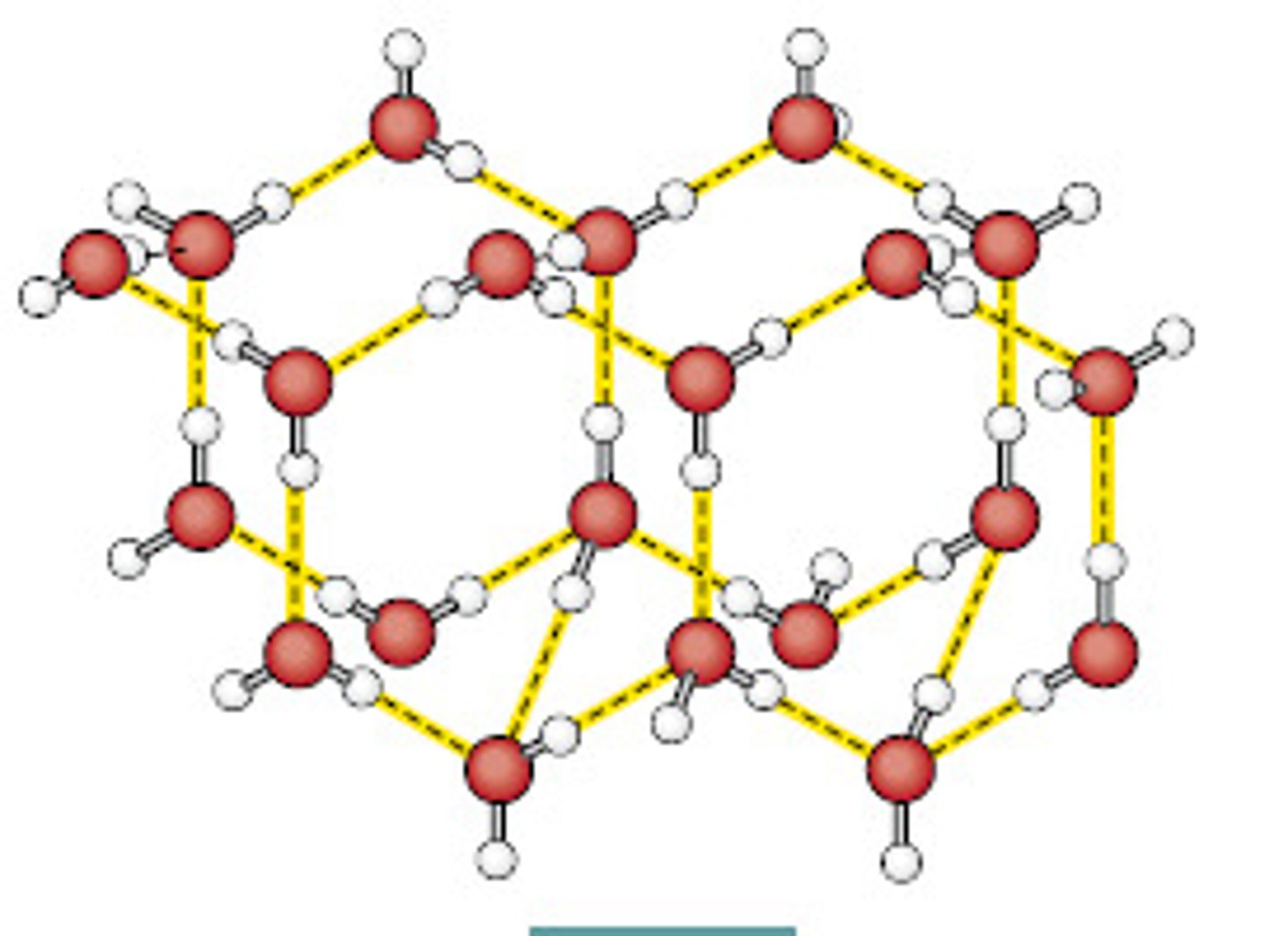
Molecular Solids
Made up of atoms or molecules held together by intermolecular forces, such as London dispersion forces, dipole-dipole interactions, or hydrogen bonds. They typically have low melting points, are flexible, and are poor conductors of electricity. A common example is sucrose (table sugar).
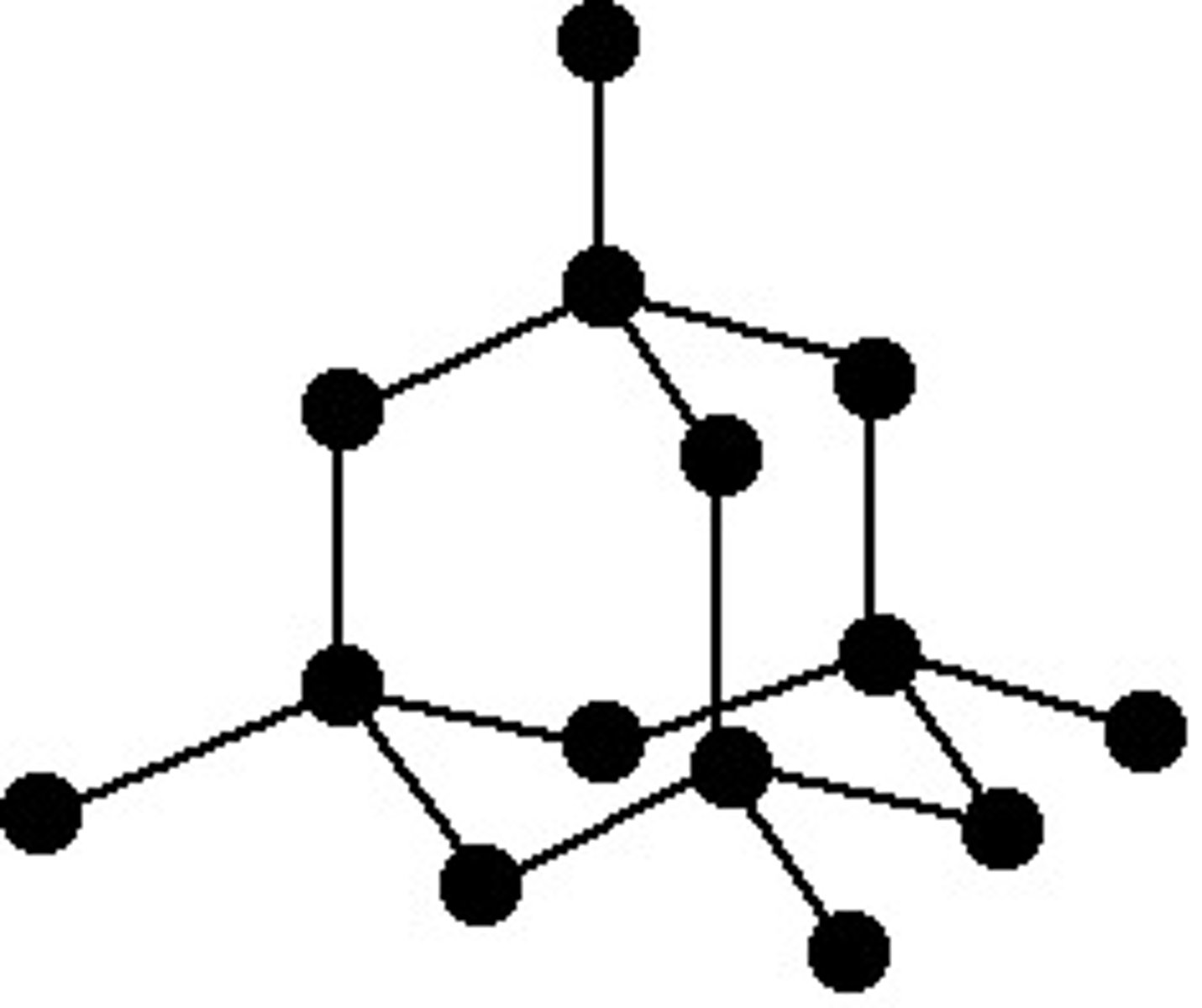
Covalent -Network Solids (Atomic)
Composed of a continuous network of atoms held together by covalent bonds. These solids have high melting points, are very hard, and generally poor conductors of electricity. Examples include diamond and graphite.
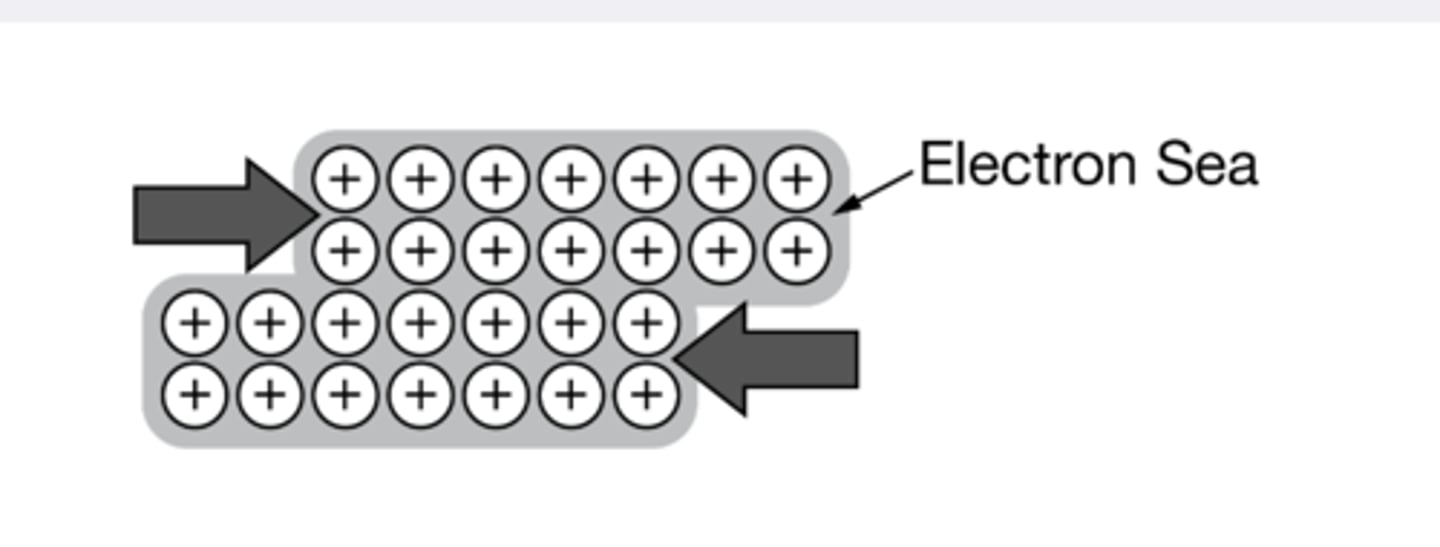
Metallic Solids
Composed of metal atoms held together by metallic bonding, where a "sea" of delocalized electrons is shared collectively among the atoms. These solids typically have high melting points, are hard, malleable, and are excellent conductors of electricity.
Melting
Solid → Liquid

Freezing
Liquid → Solid
Vaporization
Liquid → Gas

Condensation
Gas → Liquid

Sublimation
Solid → Gas

Deposition
Gas → Solid

Phase Diagram
A graphical representation of physical states of a substance under different conditions of temperature and pressure. It gives the possible combinations of pressure and temperature at which certain physical state or states a substance would observe.
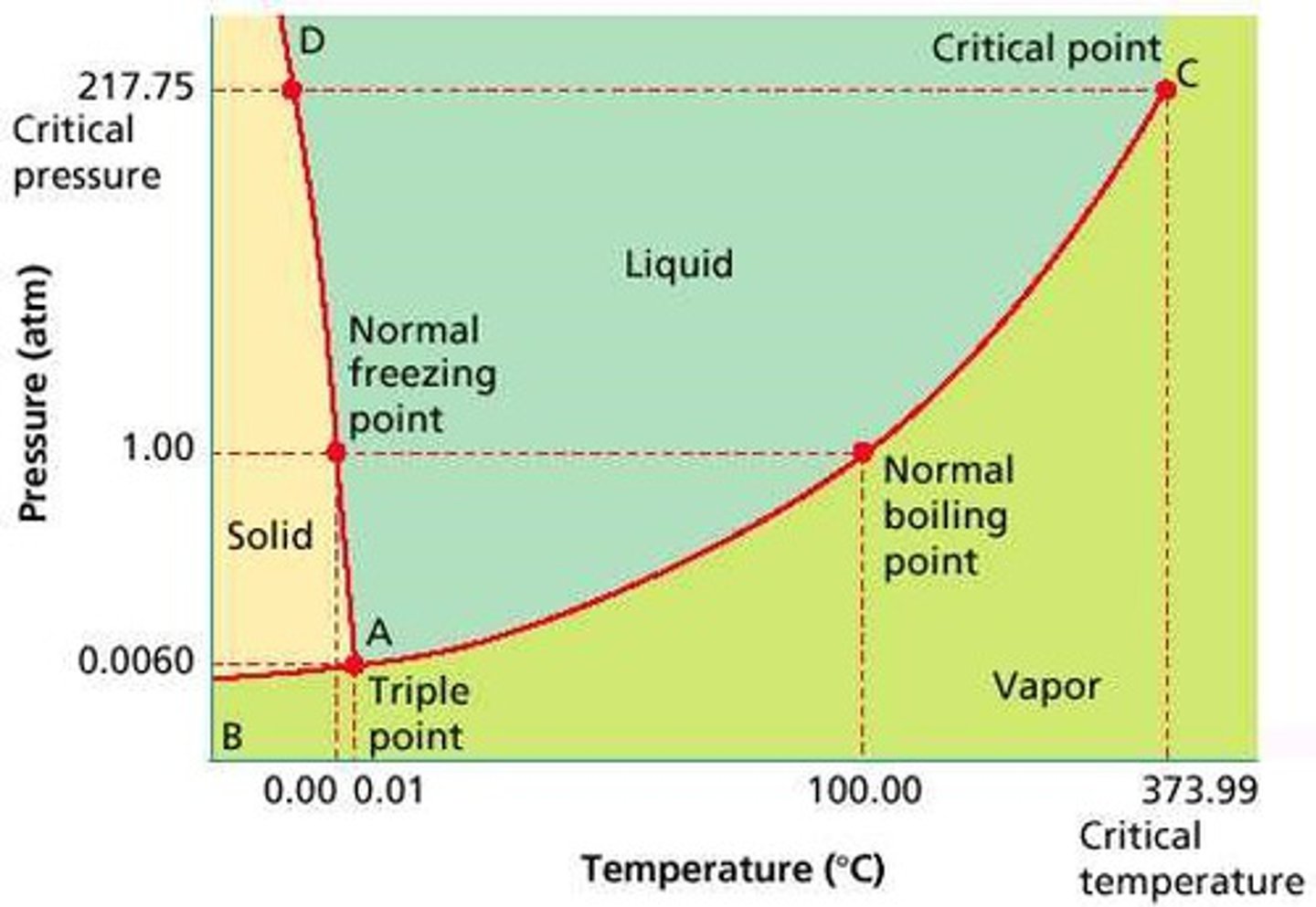
Melting/Freezing Curve
The green line on a phase diagram that divides the solid and liquid phases. It represents the melting point (solid → liquid) and freezing point (liquid → solid) of a substance.
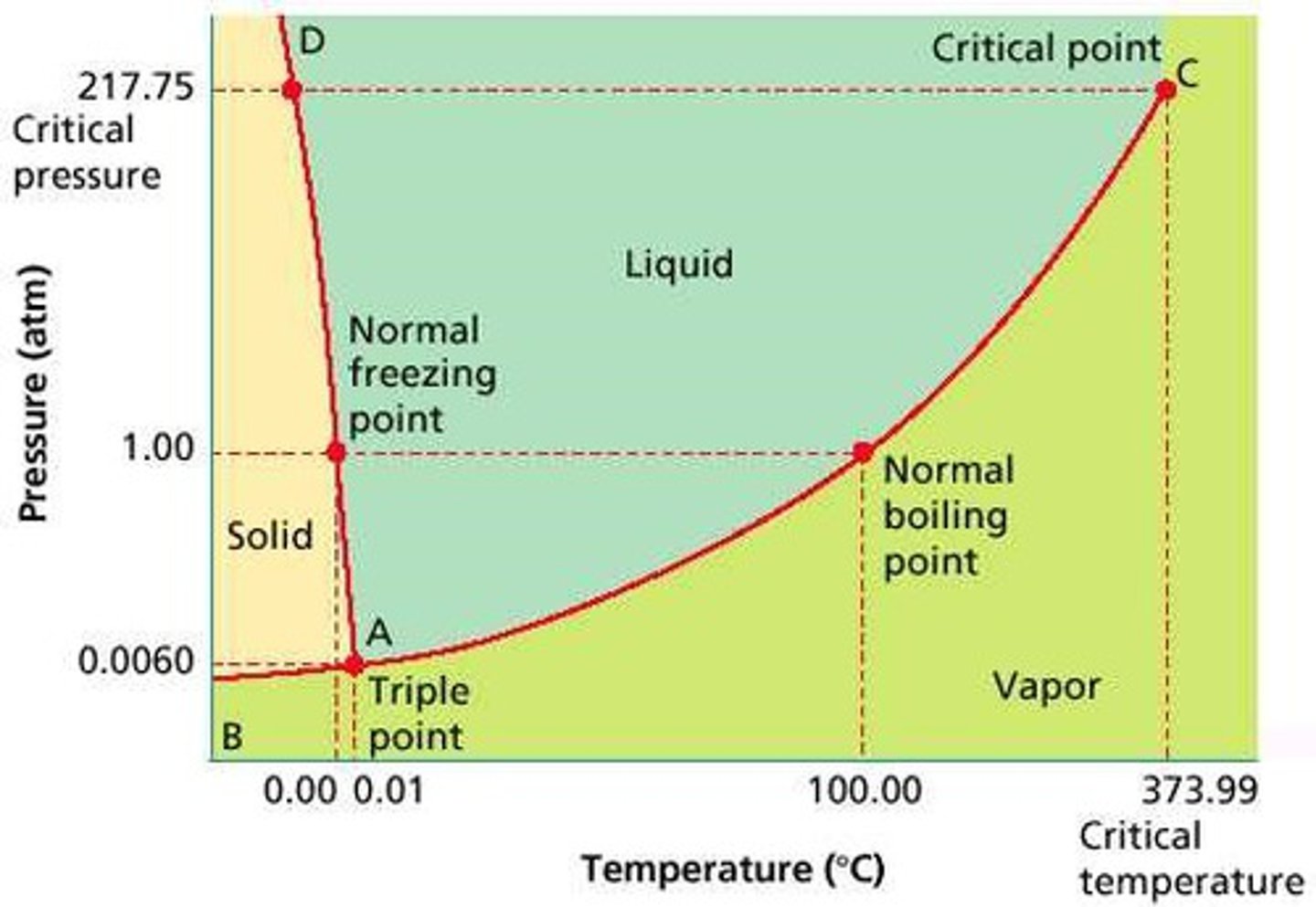
Vaporization/ Condensation Curve
The blue line divides the liquids and gas phase and represents vaporization (liquid to gas) and condensation (gas to liquid) point
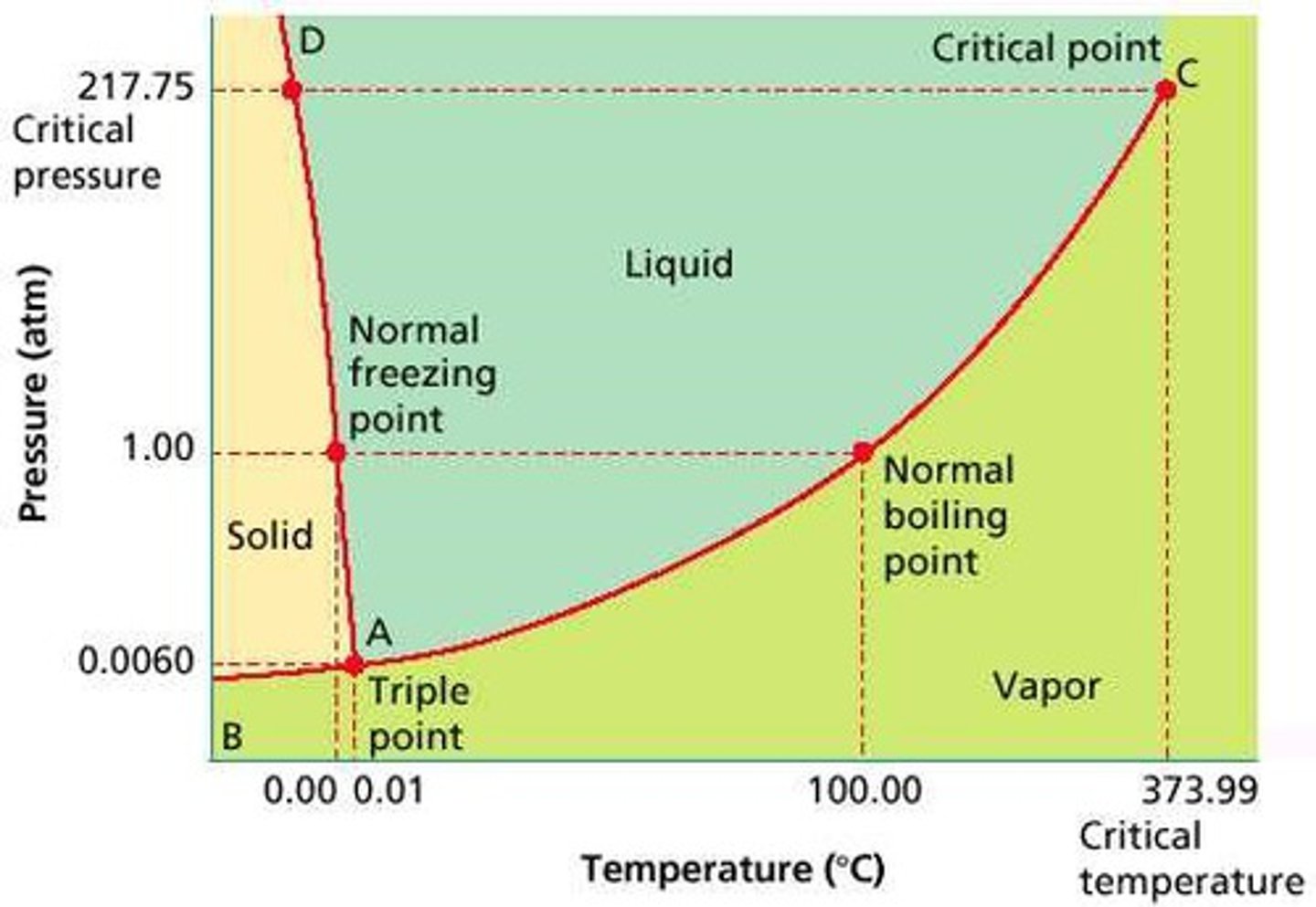
Sublimation/ Deposition Curve
The red line divides the solid and gas phases and represents sublimation (solid to gas) and deposition (gas to solid) points
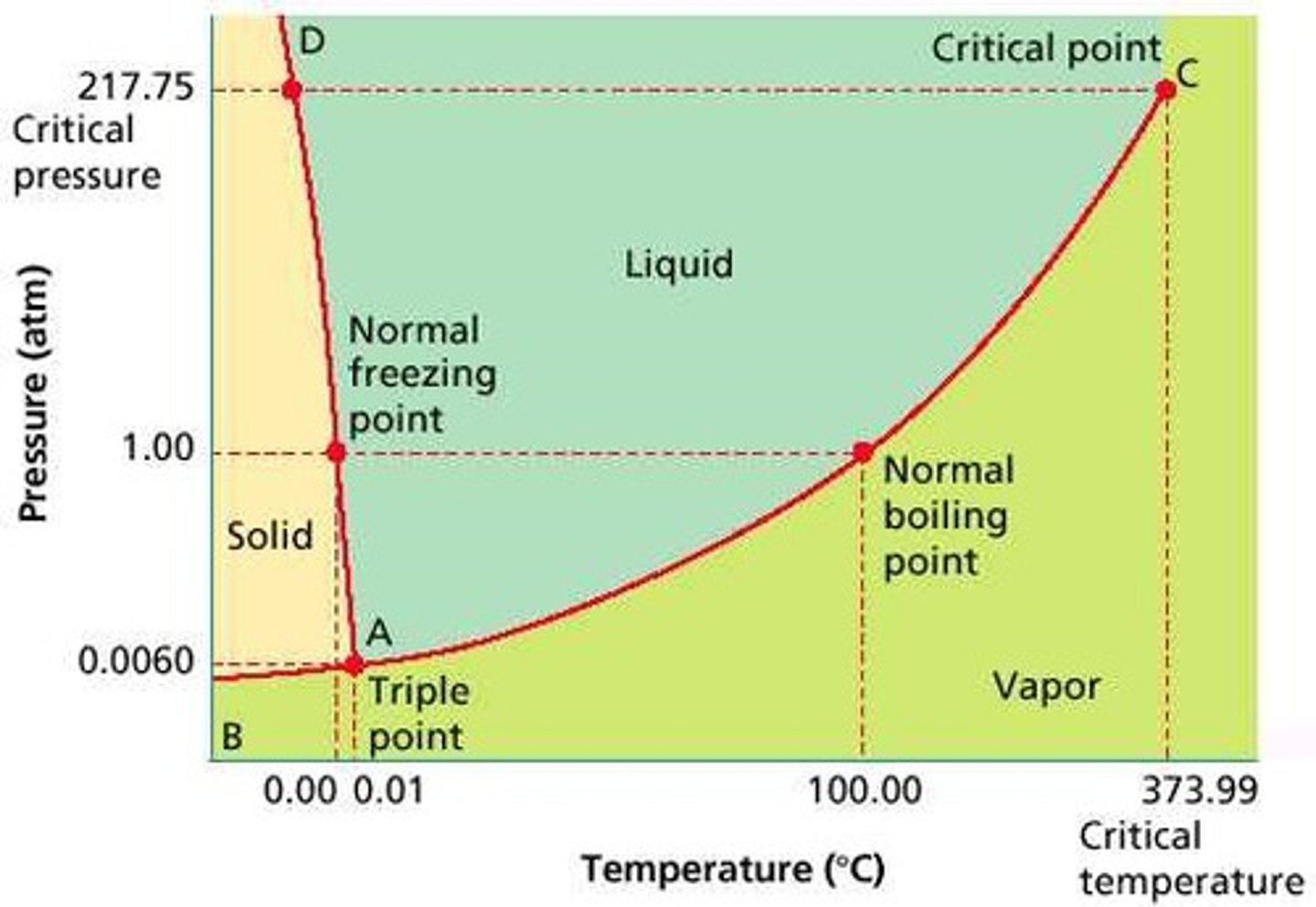
The Triple Point
Is the combination of pressure and temperature at which all three phases of matter are at equilibrium. It is the point on the phase diagram at which the three states of matter coexist. The lines that represent the conditions of solid-liquid, liquid-solids and solid-vapor equilibrium meet.
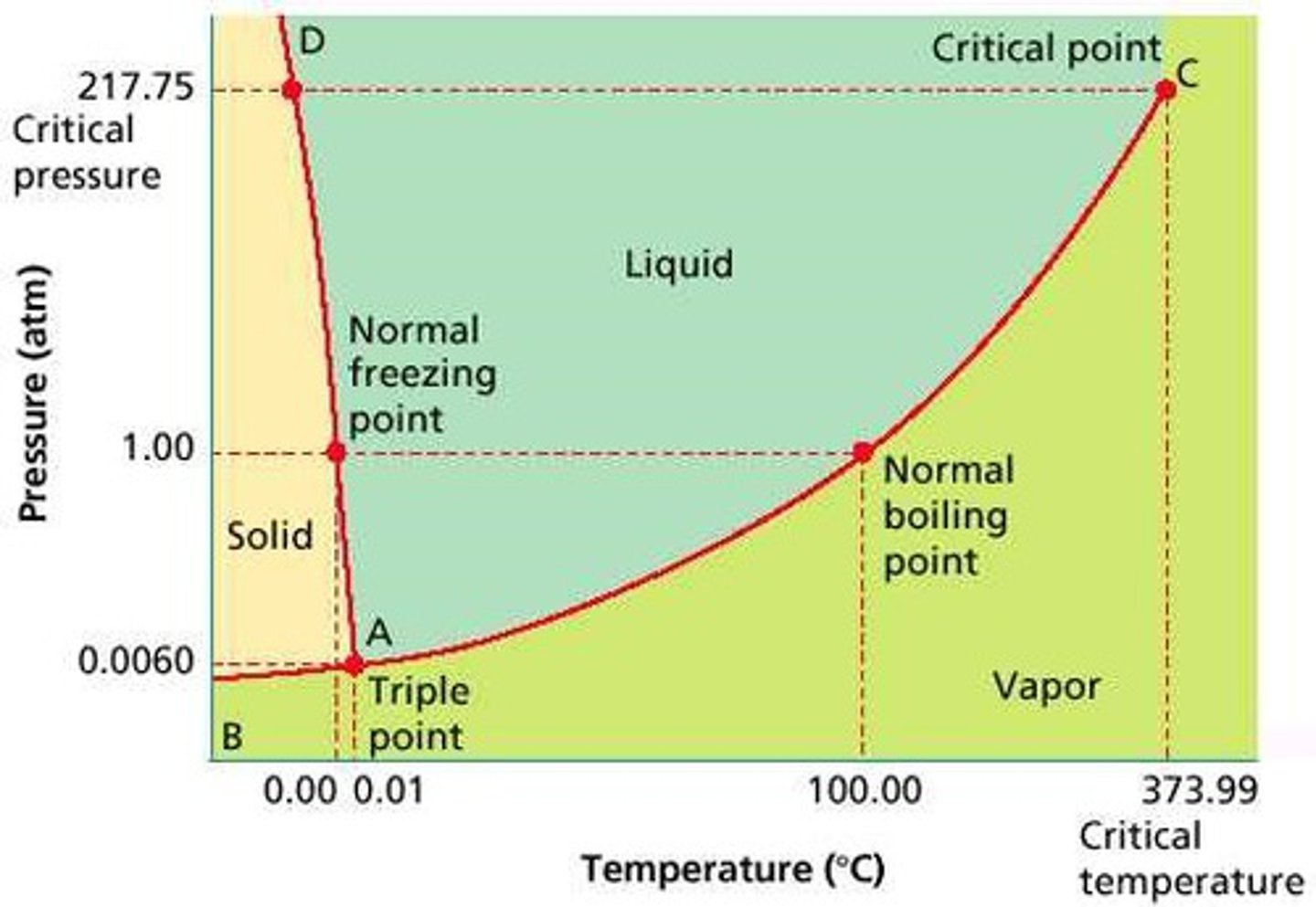
The Critical Point
The critical point terminates the liquid/gas phase line. It is the set of temperature and pressure on phase diagram where the liquid and gaseous phases of a substance merge together into a single phase. Beyond the temperature of the critical point, the merged single phase is known as a supercritical fluid.
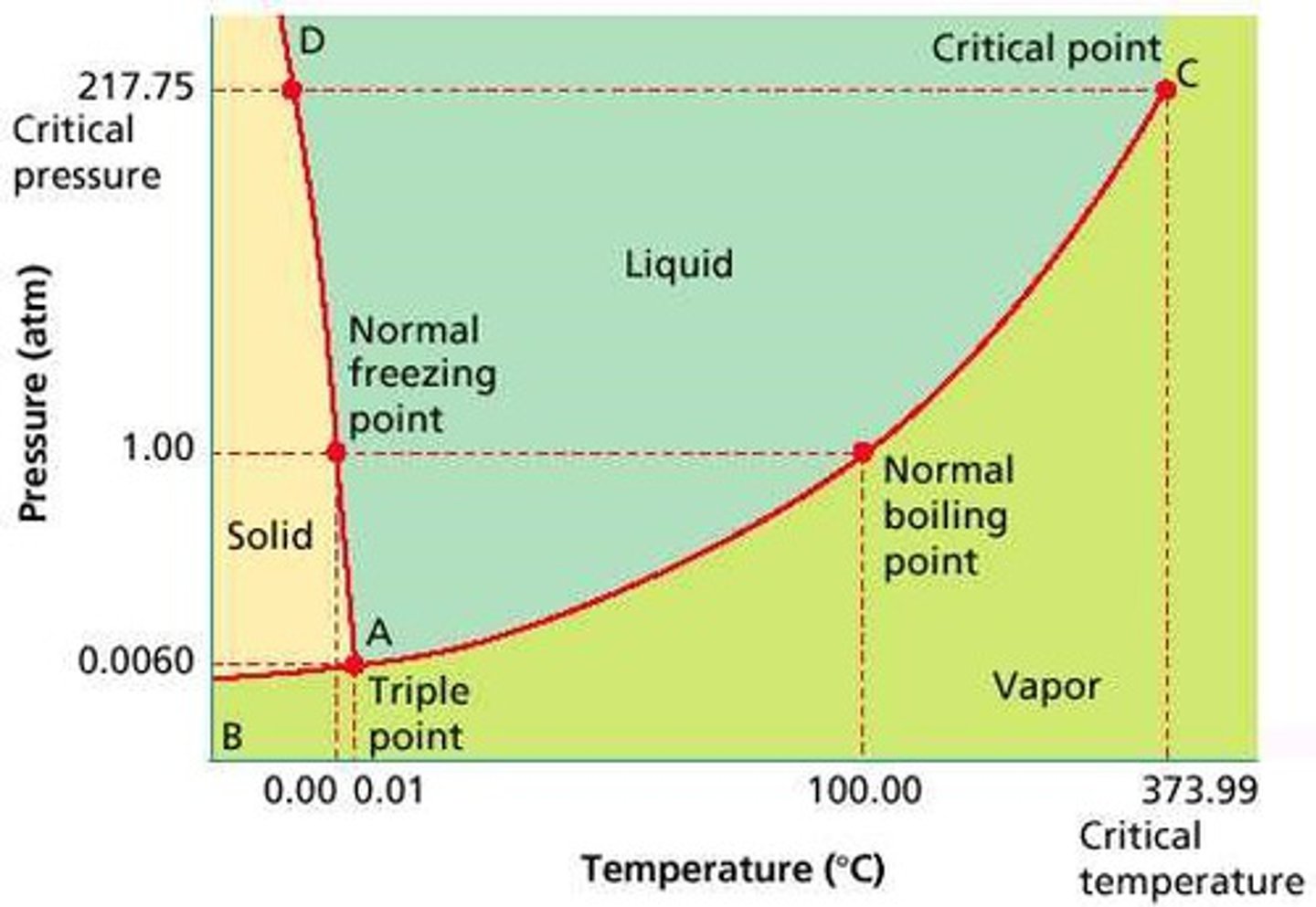
Solutions
A type of homogeneous mixture that is made up of two or more substance. Consists of solute and solvent

Dilute Solution
a solution that has very little solute in the solvent.

Concentrated Solution
a solution where the solvent has a lot of solute in the solution.

Unsaturated
contains less solute that the solvent's capacity to dissolve.
Saturated
contains the maximum amount of solute that the solvent can dissolve at a given temperature
Supersaturated
contains more dissolved solute than saturated solution.
Soluble
a substance that dissolves in a solvent

Insoluble
a substance that will not dissolve in a solvent.

Miscible
When a liquid dissolved in another liquid to form homogenous solution.
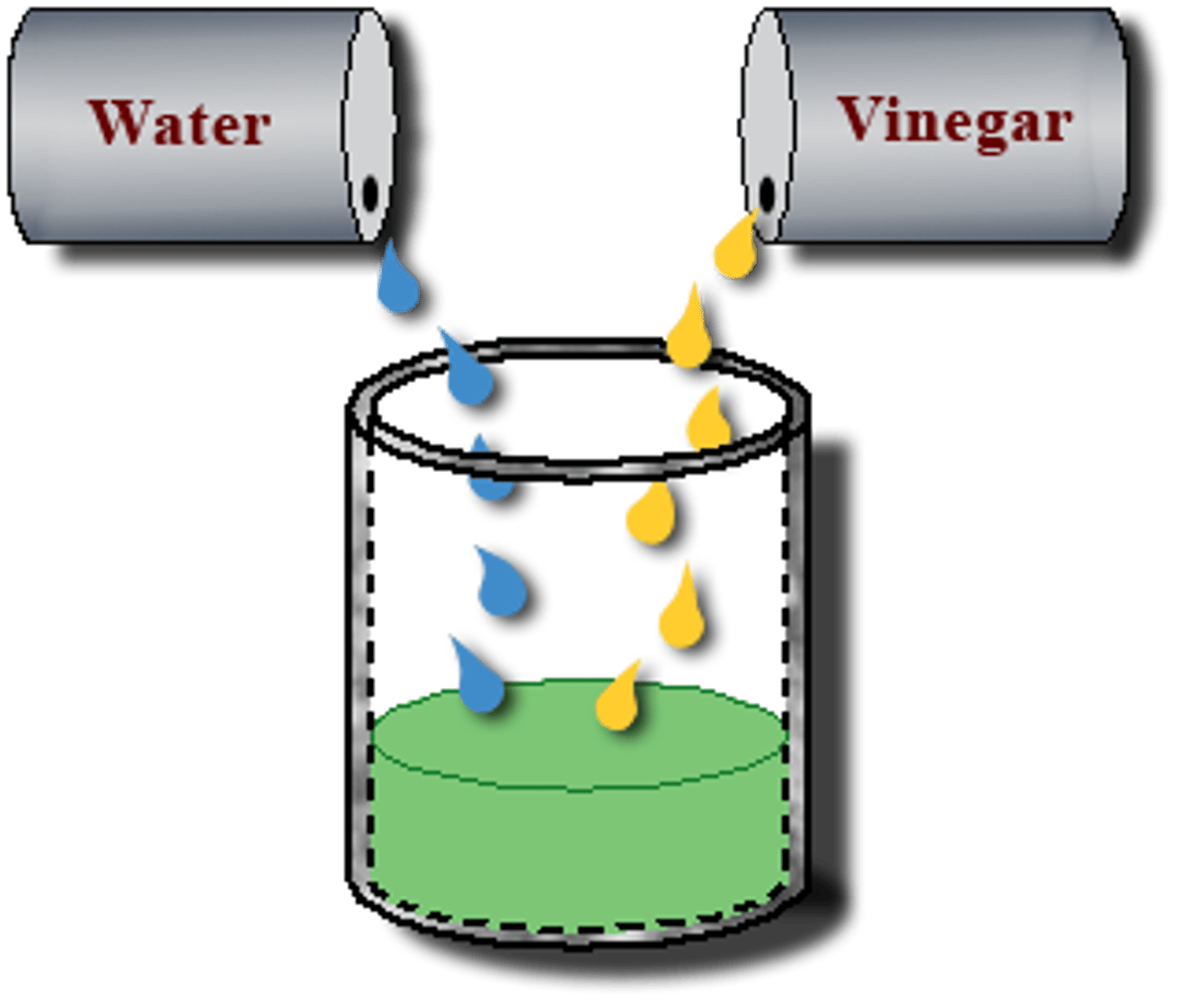
Immiscible
When two liquids do not dissolve in another liquid to form homogenous solution.
Hydrophilic Molecules
Molecules that are completely miscible with water and can form hydrogen bonds with it. Examples: Ethanol (C₂H₅OH) Acetic acid (CH₃COOH)
Hydrophobic Molecules
Molecules that do not dissolve in water. Examples: molecules of organic fats and oils; molecules present on surface of a leaf
General Properties of Solutions
A solution is a homogeneous mixture made up of two or more components. It has a variable composition, meaning the ratio of solute to solvent can vary. The dissolved solute is either molecular or ionic in size. A solution can be colored or colorless, but it is generally transparent. It remains uniformly distributed and does not settle out over time. Lastly, the solute can be separated from the solvent using physical methods.
Dilution Process
When a solute is dissolved in suitable solvent, dissolution occurs. The measure of speed of solution is called dissolution rate.
Particle Size
The smaller the size the faster the dissolution rate is.
Temperature
The higher the ___________ is the faster the dissolution rate is.
Concentration of Solution
Higher concentration of solution, lower rate of dissolution, lower solubility.
Stirring
Directly proportional to the dissolving rate because it increases exposure of solute to the solvent.
Solvation of Ionic Substances
Solvent molecules surrounds the solute ions-solvation or hydration (in case of water as solvent). Ion-dipole interactions.
Solvation of Molecular Solids
Polar molecular solids dissolve in polar solvents, while non-polar molecular solids dissolve in non-polar solvents. This follows the principle of "like dissolves like." The interaction is driven by intermolecular forces such as dipole-dipole interactions, London dispersion forces, and hydrogen bonding.
Heat absorbed or evolved during dissolving process
During the dissolving process, heat may be absorbed (endothermic) or released (exothermic), depending on the balance between solute-solute, solvent-solvent, and solute-solvent interactions. The overall heat change, called the heat of solution, is influenced by the heat of hydration and the lattice energy of the solute.