Ochem reactions
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
Halogenation of Alkenes - Addition of X₂
-reagents: Br2, Cl2
-regiochemistry: not relevant
-stereochemistry: anti
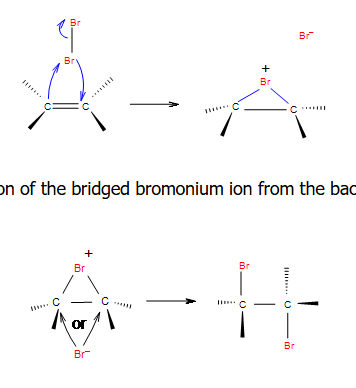
Halohydrins from Alkenes - Addition of HOX
-reagents: X2 with H2O or NBS (gives Br and OH as products)
-regiochemistry: not relevant
-stereochemistry: anti
Hydration of Alkenes - Addition of H₂O by Oxymercuration
-reagents: 1. HG(OAc)2, H2O 2. NaBH4
-regiochemistry: Markovnikov
-stereochemistry: not relevant (sometimes goes to the back)
Hydration of Alkenes - Addition of H₂O by Hydroboration
-reagents: BH3, THF (gives BH2 substituent) or BH3, THF, H2O2, NaOH, and H2O (gives OH sub)
-regiochemistry: Anti-Markovnikov
-stereochemistry: not relevent
Reduction of Alkenes - Hydrogenation
-reagents: H2 and Pt
-regiochemistry: not relevant
-stereochemistry: syn
Oxidation of Alkenes - Epoxidation
-reagents: MCPBA, (CH3)2CO or MMPP, CHCL3 (gives epoxide, always comes towards the screen) or MCPBA H2O, and H3O or OH (gives diol)
-regiochemistry: not relevant
-stereochemistry: anti (for diol)
Oxidation of Alkenes - Hydroxylation
-reagents: OsO4 and H2S, or OsO4, NaHSO4, and H2O
-regiochemistry: not relevant
-stereochemistry: syn
-produces two OH bonds
Oxidation of Alkenes - Cleavage to Carbonyl Compounds
-reagents: O3 with reductive workup like Zn/H3O or KMnO4 with heat (acid) (makes CO2 if pi bond is terminal)
-regiochemistry: N/A
-stereochemistry: N/A
Addition of Carbenes to Alkenes - Cyclopropane Synthesis
-reagents: CH2 (carbene), hv with Zn, Cl or CCl2 (carbenoid)
-regiochemistry: N/A
-stereochemistry: cis- both wedged or hashed, trans- one hash, one dash
Preparation of Alkynes - Elimination Reactions of Dihalides
-reagents: NaNh2, NH3, H2O (bases), might need extra if terminal
-regiochemistry:
-stereochemistry:
Reactions of Alkynes - Addition of HX
-reagents: HX
-regiochemistry: Markovnikov
-stereochemistry: anti
Reactions of Alkynes - Addition of X2
-reagents: X2
-regiochemistry: Markovnikov
-stereochemistry: anti
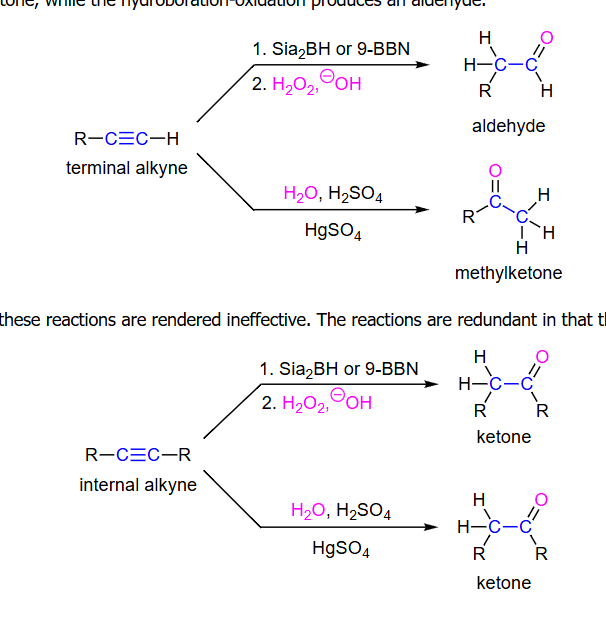
Hydration of Alkynes
-reagents: listed on image
-regiochemistry: anti (Sia)
-stereochemistry: syn (Sia)
Reduction of Alkynes 1
-reagents: H2, Pd/C
-regiochemistry: N/A
-stereochemistry: turns into trans alkane
Reduction of Alkynes 2
-reagents: H2, Lindlar catalyst
-regiochemistry: N/A
-stereochemistry: turns into cis alkene
Reduction of Alkynes 3
-reagents: Na/NH3
-regiochemistry: N/A
-stereochemistry: turns into trans alkene
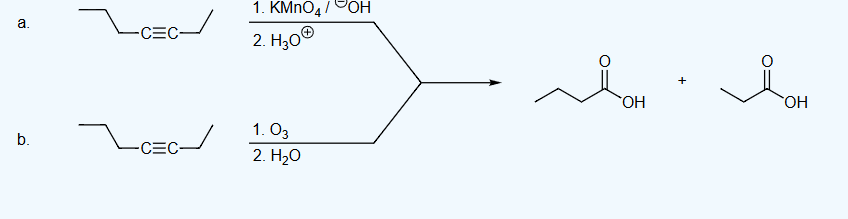
Oxidative Cleavage of Alkynes
-reagents: O3 with H2O or KMnO4/OH in H3O
-terminal alkyne will make CO2
-if there are no O’s in the reagents (besides O3 or KMnO4) then no OH group
Alkylation of Acetylide Anions
-reagents: NaNH2/NH3 and XC…….. or RRO with H3O
-needs alkyne and halide to work
-bonds to triple bond end
-can add in parts