Lecture 1 Body Fluid Compartments & Cell Membrane Transport
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
74 Terms
total water in body = __________ __________ __________
total body water
True or false: Depending on species, total body water will change.
true
How is the amount of water related to fat?
inversely
- more fat = less water
- muscles are made up of ALOT of water
- leaner the animal, less fat = more water
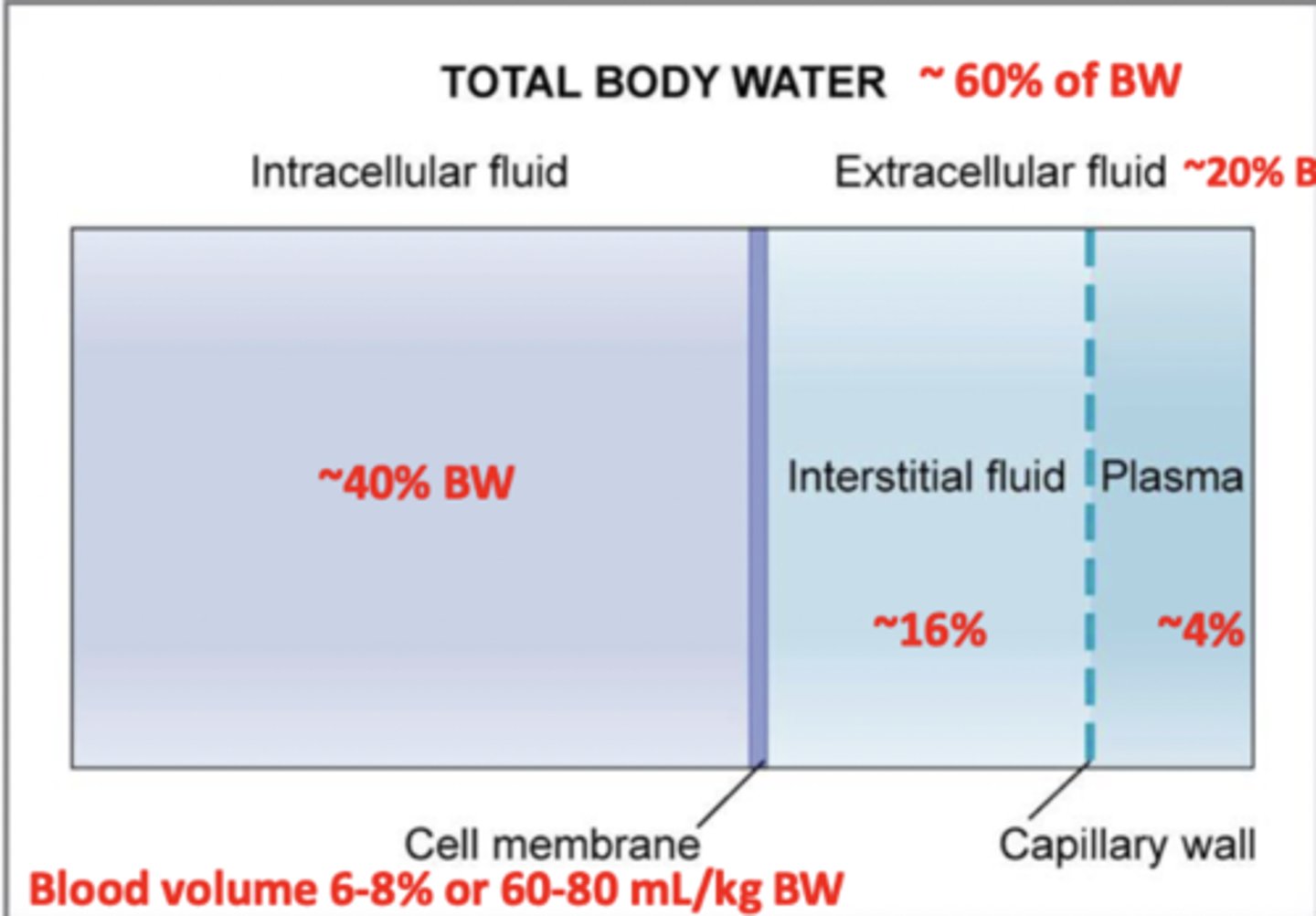
What are two major fluid compartments of the body?
intracellular fluid (ICF) and extracellular fluid (ECF)
What two components make up the extracellular fluid?
plasma and interstitial fluid
Newborns can have up to what percent of total body water?
90%
Total body water is about what percent of body weight?
What is the range?
60%; 50-70%
Intracellular fluid is about what percent of body weight?
40%
Extracellular fluid is about what percent of body weight?
Interstitial fluid is what percent?
Plasma is what percent?
20%; 16%; 4%
Blood volume is about what percent of body weight?
6-8%
The average blood volume is about what portion of body weight?
70 mL/kg
Range is 60-80 mL/kg BW
How much total body water does a 20 kg dog have?
20 kg x 0.60 = 12 L or 12,000 mL
make sure you have volume units on the answer
1 L of water is about how many kilograms?
1
interstitial fluid is made up of what kind of cells?
tissue fluid bathing cells
True or false: Fluid compartments differ in concentrations of various solutes
True
What do you measure AMOUNTS in?
moles, equivalents, or osmoles
amount of charged solute; number of moles of solute multiplied by its volume
equivalent
CaCl2 = 2 Eq of Ca and Eq of Cl
Ca = valence 2+
1 mole of calcium = 2+
4 Eq
number of particles into which a solute dissociates in solution
osmole
sodium chloride = salt; dissociates in 2 particles, 2 osmoles in that solution
True or false: Osmoles contribute to osmotic pressure.
True
What do you measure CONCENTRATIONS in?
mol/L, mmol/L, mEq/L, Osm/L, or mOsm/L
Osmolarity = __________/__________
osmoles/L
What expresses H ion concentration?
pH
True or false: H ion concentration in body fluids is relatively low.
true
pH = _____________
-log10[H+]
Each body fluid compartment must have the same concentration of cations as of anions which is called?
Electroneutrality
What is the major cation in the ECF?
Na+
What are the major anions in the ECF?
Cl-, HCO3- (bicarbonate)
What is the major cation in the ICF?
K+
What are the major anions in the ICF?
Proteins and organic phosphates
ICF has low __________ __________ and is more __________.
ionized Ca2+; acidic
free calcium and biologically active calcium
ionized Ca2+
Ionized calcium and bound calcium
Total Ca2+
True or false: Osmolarity is the same in the ECF and the ICF; therefore, electroneutrality is maintained.
true
What is the normal osmolarity of the ECF and the ICF?
290-300 mOsm/L
cell membranes are not freely soluble to all solutes
selectively permeable
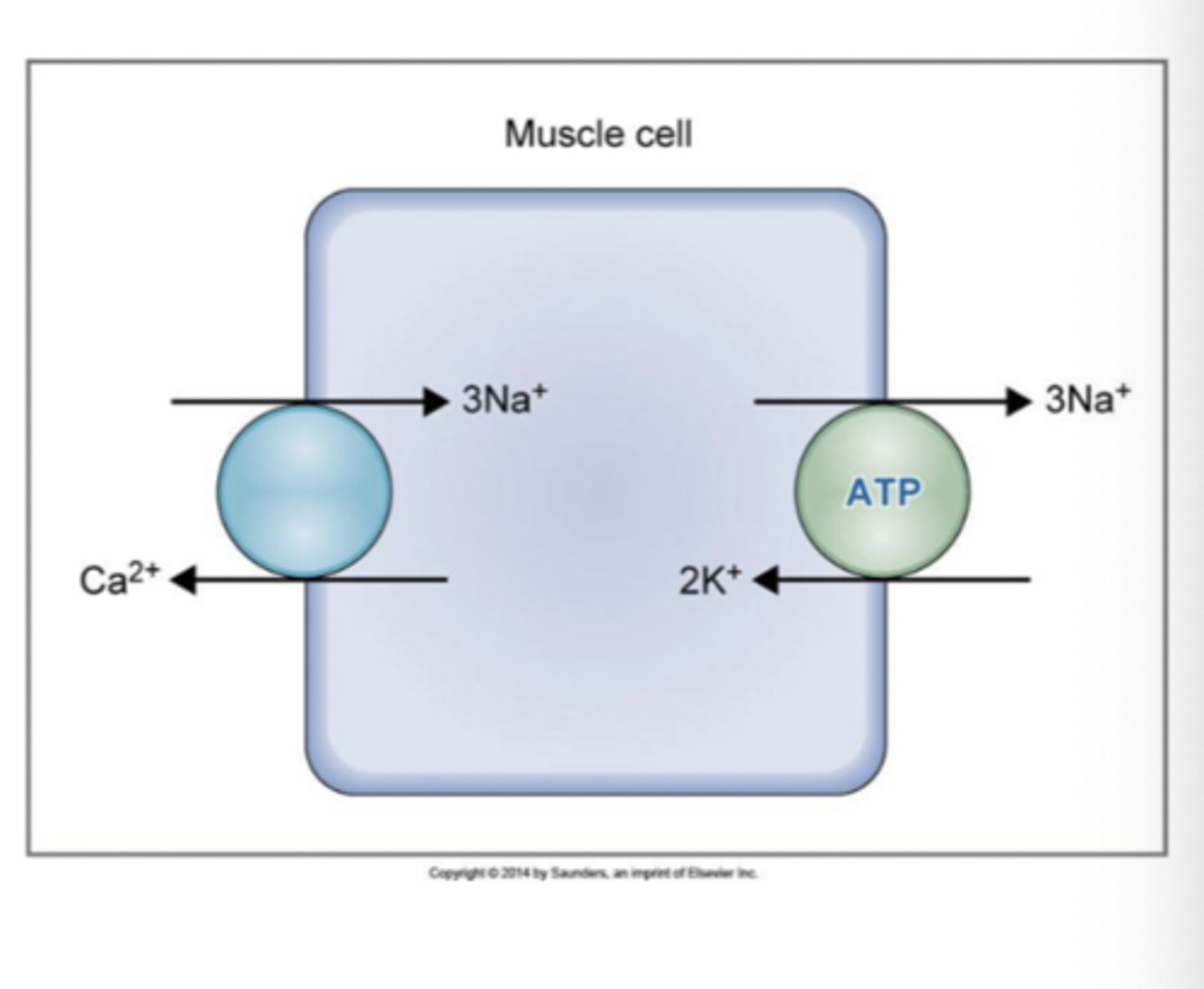
Which two transport mechanisms directly use ATP?
Na+/K+ ATPase pump and Ca2+/ATPase pump
What does the Na+/K+ ATPase pump do?
pumps Na+ out of the cell and K+ into the cell
What does the Ca2+/ATPase pump do?
pumps Ca2+ out of the cell
Why are ion concentration differences important?
Allows nerve and muscle cells to have resting membrane potentials due to?
K+ difference
Why are ion concentration differences important?
Upstroke of action potentials in nerve and muscle cells, and absorption of nutrients due to?
Na+ difference
Excitation-contraction coupling in muscle cells depends on?
Ca+ difference
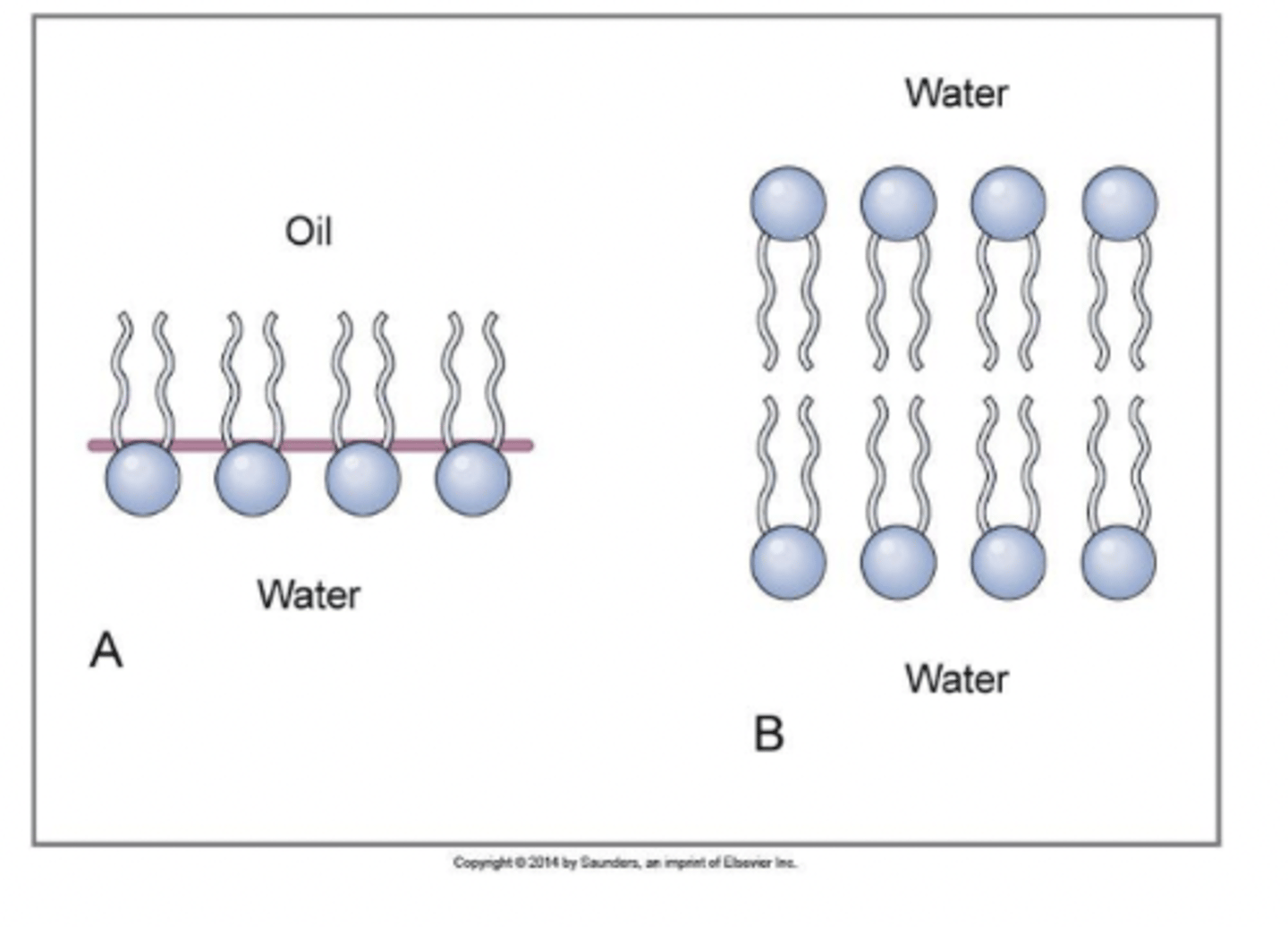
What are cell membranes composed of?
lipids and proteins
allow cell membrane to be permeable to lipid soluble substances (CO2, O2, fatty acids, steroid hormones)
lipids
phospholipids, cholesterol, and glycolipids are all examples of what?
lipids
make up transporters, enzymes, hormone receptors, antigens, and ion and water channels in the cell membrane
proteins
helps to form a lipid bilayer in cell membrane
phospholipid component
What part of the phospholipid is water soluble?
What part is lipid soluble?
How can this be described?
glycerol backbone;
FA tails;
amphipathic = having both hydrophillic and hydrophobic parts
What types of specific proteins make up the protein component of the cell membrane?
integral proteins and peripheral proteins
can be transmembrane proteins (hormone or neurotransmitter receptors, pores, ion channels)
integral proteins
proteins that span the entire membrane
transmembrane proteins
proteins not bound to the membrane, are loosely attached by electrostatic interactions, and are only on one side
peripheral proteins
True or false: Simple diffusion is not carrier mediated.
true
What are the two main ways that transport can occur across cell membranes?
D
A
down an electrochemical gradient
against an electrochemical gradient
What are the two types of transport down an electrochemical gradient?
S
F
simple diffusion
facilitated diffusion
True or false: There is no input of energy in simple or facilitated diffusion.
true
Which type of diffusion depends on the concentration difference?
simple
Which type of diffusion depends on a carrier protein?
facilitated
2 types of transports that occur against an electrochemical gradient
primary transport
secondary transport
primary transport uses a _______ input of energy
direct
secondary transport uses an _________ input of energy
indirect
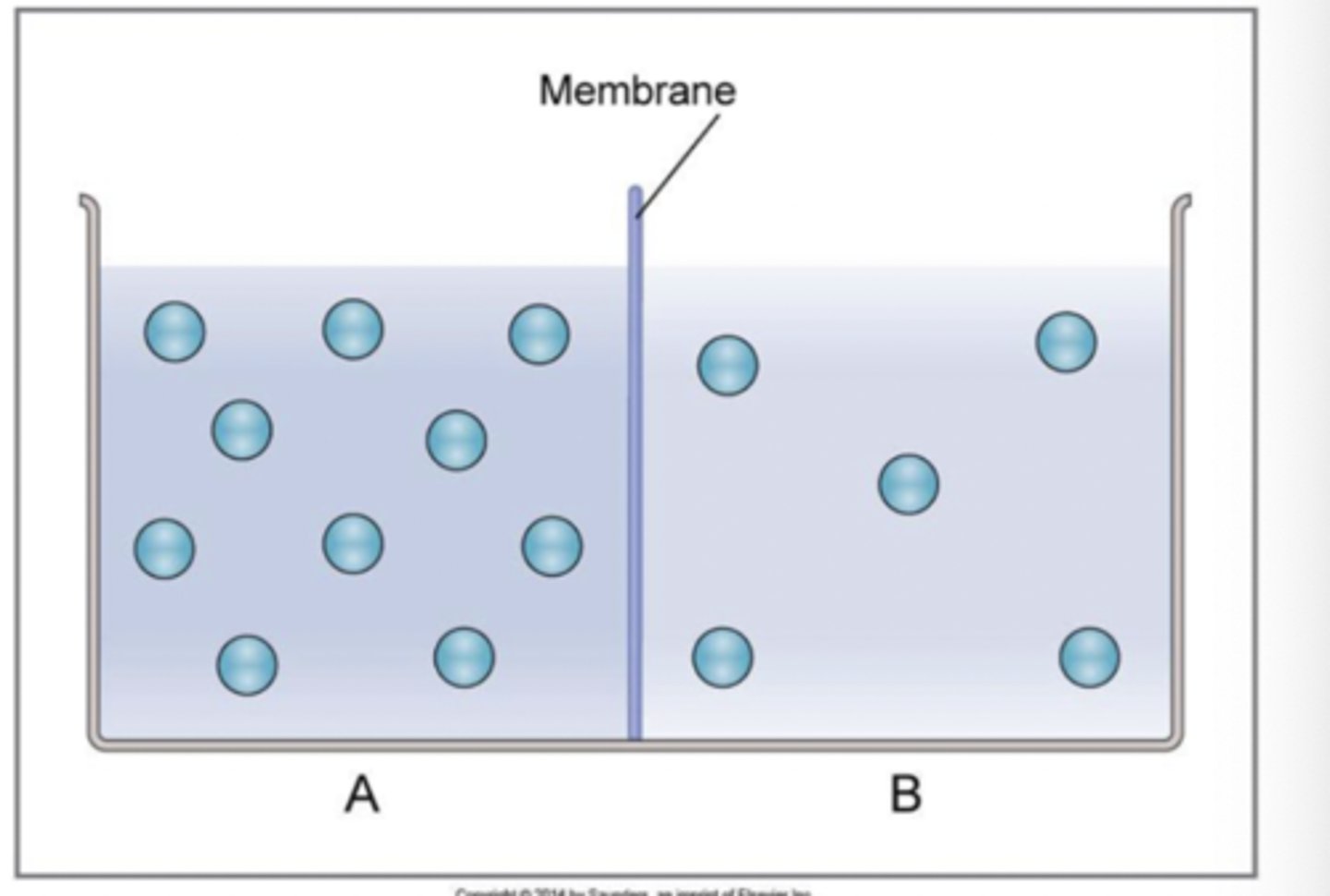
true or false: when two solutions are separated by a membrane permeable to the solute, the solute will equilibrate across the membrane
true
in example, net diffusion of solute from A to B because initially, more solute in solution A
movement of solute depends on what 5 factors?
1. concentration gradient - driving force, bigger concentration difference, greater driving forcer
2. partition coefficient - based on lipid solubility of solute, greater the lipid solubility of our solute, easier it can diffuse
3. diffusion coefficient - based on size of solute and viscosity of solution, very small solutes moving through a nonviscous solution diffuses easier
4. thickness of membrane, harder to diffuse when membrane thickness increases
5. surface area - greater surface area = higher diffusion rate ]
(2-5 relate to permeability)
what are two additional consequences of charge on an ion that is diffusing?
1. a potential differecne across a membrane will alter the rate of diffusion of a charged solute
2. a diffusion potential can be created when a charged solute diffuses down its concentration gradient
true or false: diffusion of a positively charged ion will slow down if diffusing into an area of positive charge
true
Simple or facilitated diffusion?
- uses a carrier protein
- no input of energy
- because of limited # of carriers, it will proceed faster at relatively low solute concentrations, lots of solute = rate will slow down
Facilitated
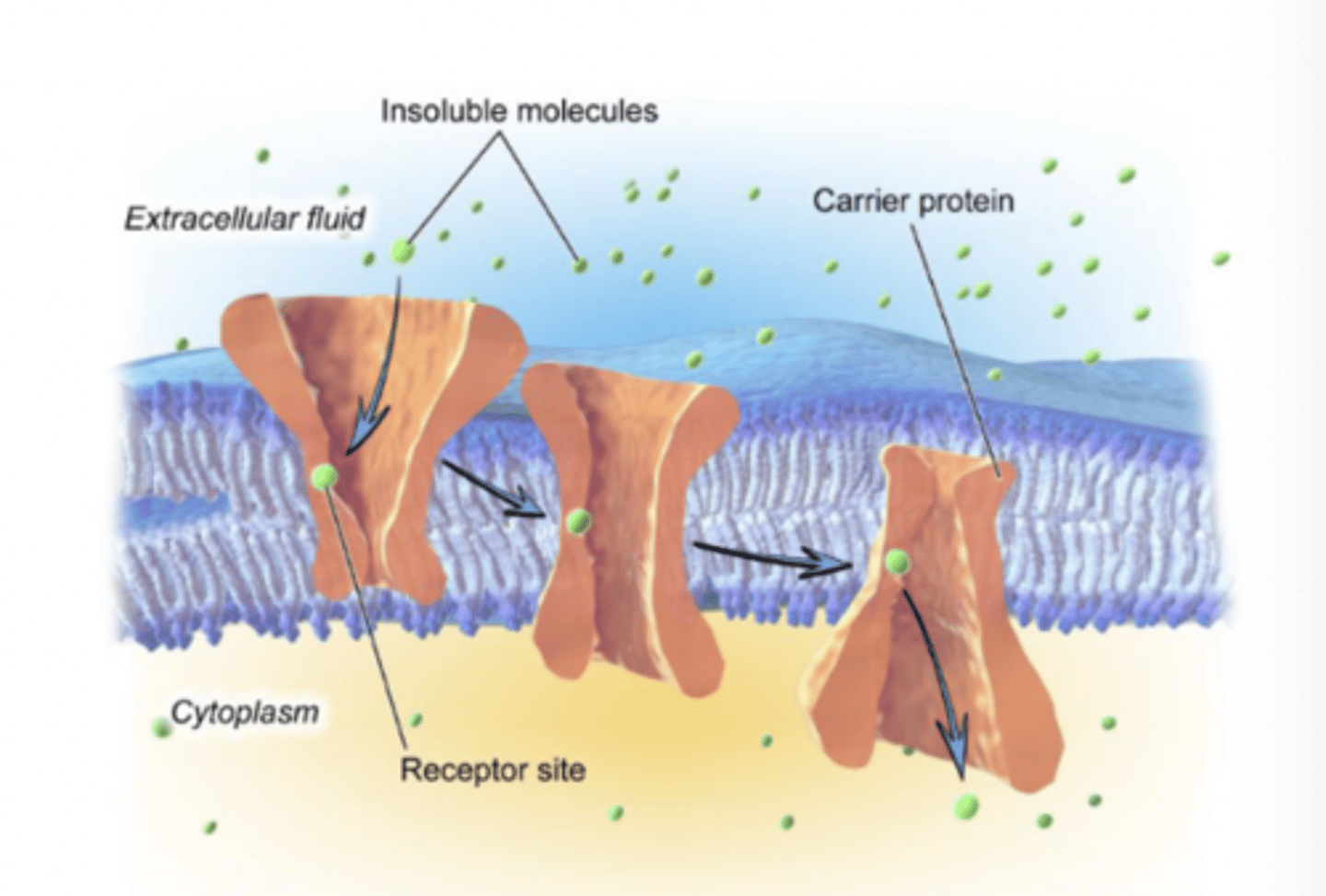
Example of facilitated diffusion?
GLUT 4 transporter in skeletal and adipose tissue
- transports glucose into cell
- D-galactose also competes for binding
True or false: facilitated diffusion levels off at saturation (binding sites) while simple diffusion keeps going and can keep going as long as there is a concentration gradient
True
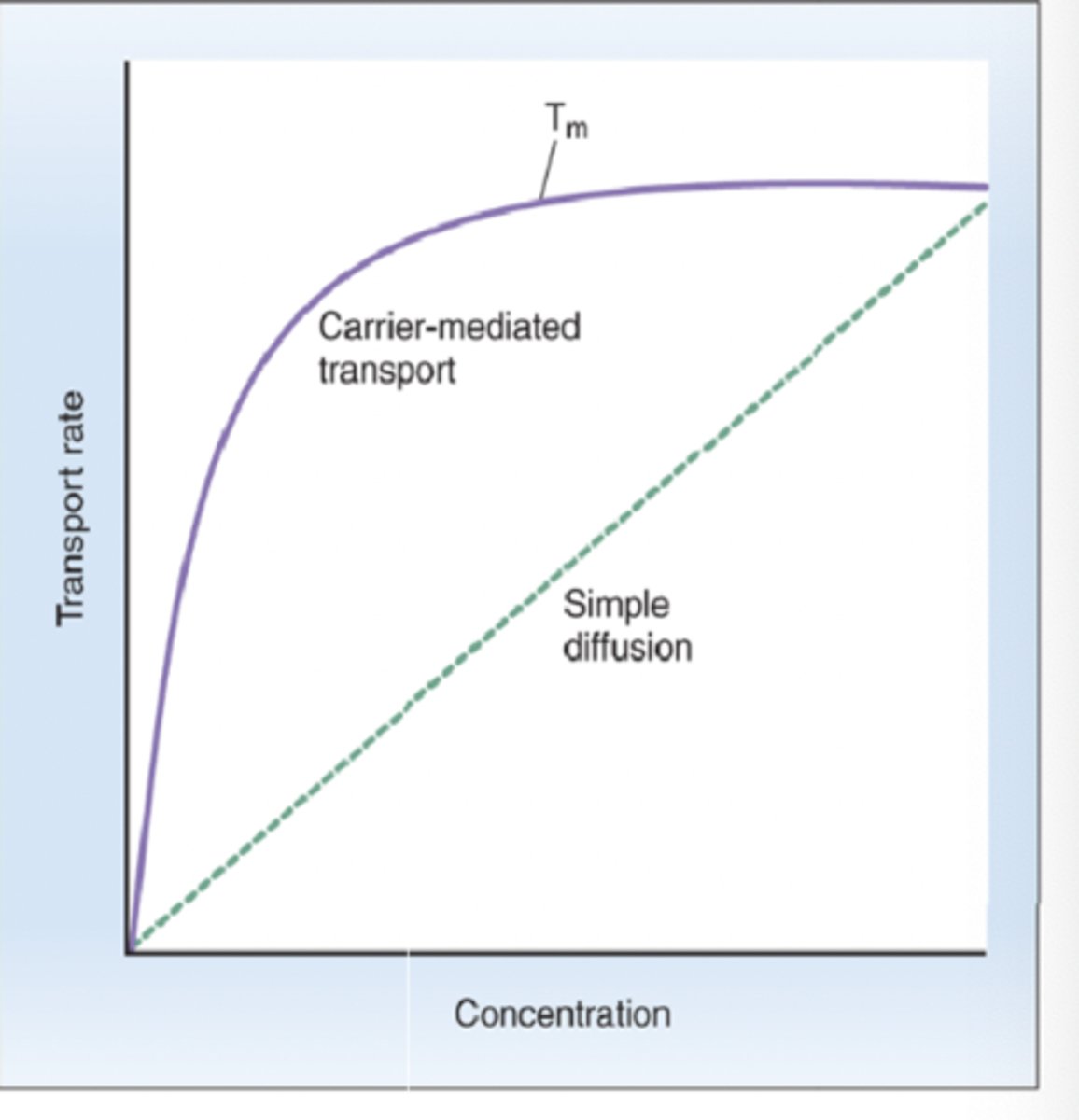
3 features of carrier-mediated transport
1. Saturation
- carrier proteins have limited # of binding sites for solute, rate of transport increases at a higher rate at lower solute concentrations, transport levels off as concentration increases
2. Stereospecificity
- binding sites for solute on carrier proteins are specific
3. Competition
- although binding sites are specific, carriers may recognize and bind chemically-related solutes
What is primary active transport?
one or more solutes moved against a concentration gradient directly using ATP
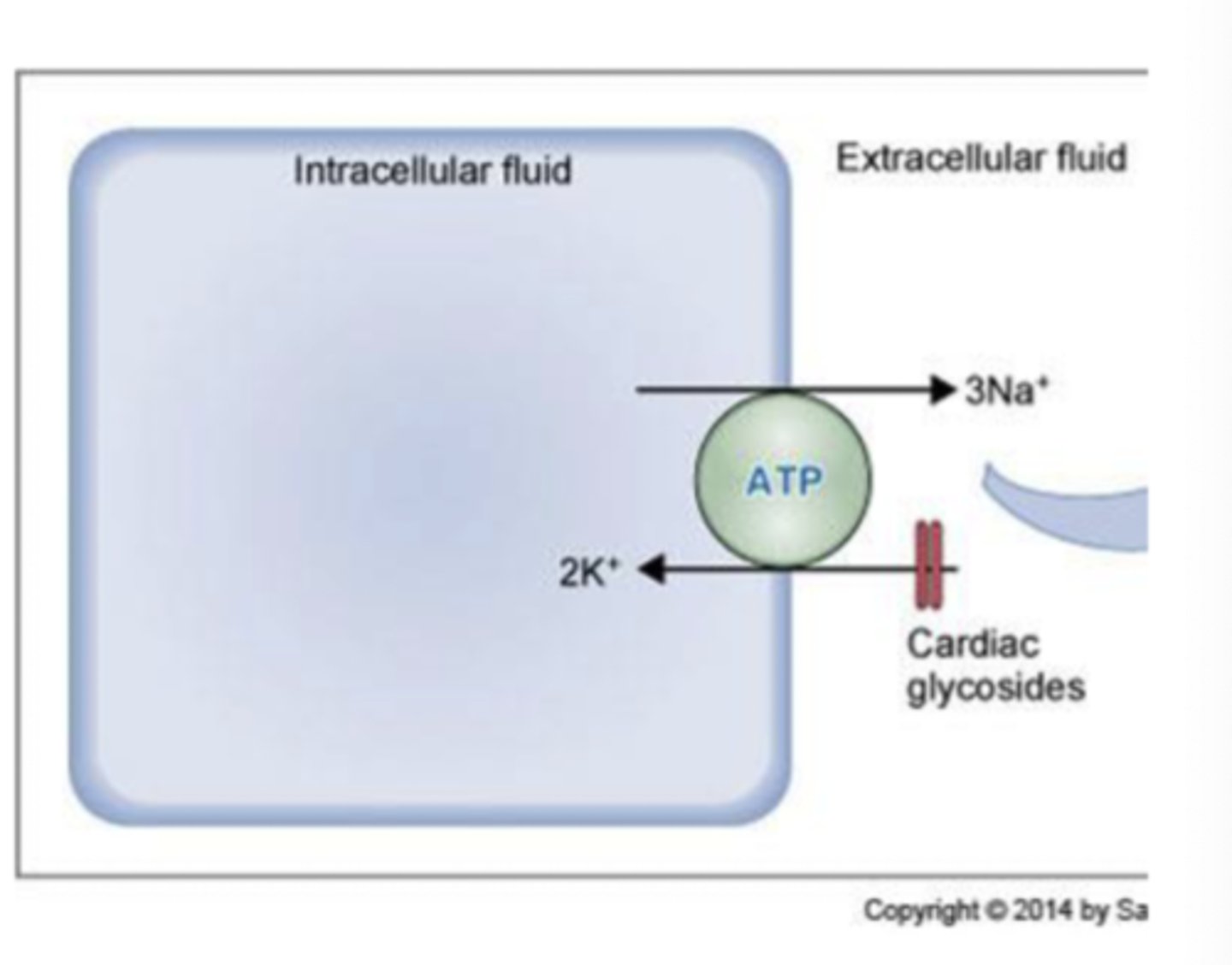
3 pumps related to primary active transport
1. Na+/K+ ATPase pump
- present in membranes of ALL cells
- 3 Na+ pumped to ECF and 2 K+ pumped to ICF creates a charge separation and potential difference
- cardiac glycosides inhibit this protein transporter
2. Ca2+ ATPase pump
- plasma-membrane Ca2+ ATPase (PMCA), one Ca out for every ATP used
- sarcoplasmic and endoplasmic reticulum Ca2+ ATPase (SERCA), 2 Ca from ICF into Sr or ER for every ATP
3. H+/K+ ATPase pump
- parietal cells of gastric mucosa - pumps H+ into lumen of stomach
What is secondary active transport?
indirectly uses energy by utilizing the Na+ gradient to transport solutes against their concentration gradient (low to high)
2 types of transports related to secondary active transport?
1. co-transport (symport)
2. counter-transport (antiport)
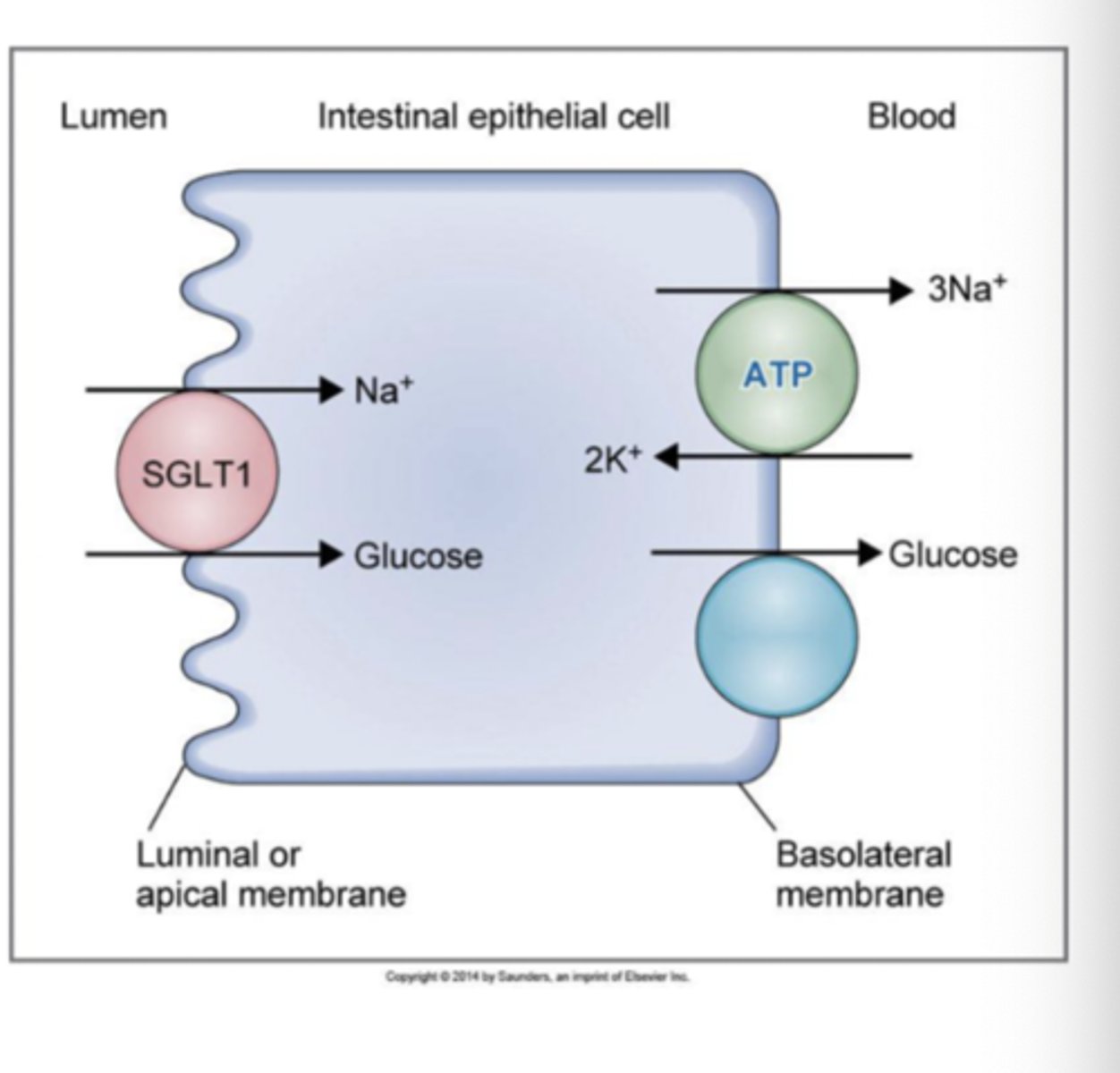
What is co-transport?
all solutes transported in same direction
- Na+/glucose co-transporter (SGLT1)
- Na+/amino acid co-transporter
- Na+/K+/2Cl co-transporter in renal tubule
*intestine & renal tubule)
What is counter-transport?
solutes move in opposite directions
Na+ moves into cell and other solutes move out of cell
- Ca2+/Na+ exchnage
- Na+/H+ exchange