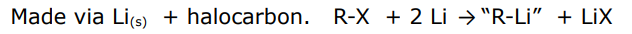20312 - core inorganic chemistry
1/410
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
411 Terms
what is wrong with assuming that shielding is only based on n
s orbitals are more penetrating than p and there is some probability of 2s electrons being close to the nucleus
describe how Zeff differs along a period
it increases left to right and increases down a group
explain the trend for Zeff across a row
electrons with the same n values are relatively poor at screening both s and p orbitals decrease in energy across a row so Zeff increases
describe covalent radii in a periodic table
Radii are expected to decrease across a period as Zeff increases from left to right
Radii increase down a groups
explain the trend for covalent radii across a period
electrons in the same shell are poor at shielding/ screening nuclear charge
explain the trend for radii down a group
increases down the group as n is the dominant effect over Zeff
describe the trend of ionic radii in the periodic table
ionic radii follows similar trends to covalent but anions are larger due to a lower Zeff and greater interelectron repulsion, conversely cations are smallet
describe the trend in ionisation energies in a periodic table
IE generally decreases down a group due to the valence orbital becoming higher in energy 1st IE generally increases left to right across a period as Zeff increases but not uniformly
define ionisation energy
the energy required to remove an electron from the gaseous atom or molecule in its ground state
what are the exceptions in IE trend across a period
group 13 has a lower IE than 12 or Mg>Al group 16 also has a lower IE than 15 P>O
explain the trend in IE between group 12 and 13
3p orbitals are of a higher energy than 3s so it is slightly easier to remove an electron from 3p than 3s
Explain the trend between IE for group 15 and 16
np3--> np4 pairing energy 2 electrons in one orbital leads to more Coulombic repulsion lowering IE
what is exchange energy
describe the number of ways of exchanging pairs of equivalent spin electrons in a configuration
describe how exchange energy and IE interact
exchange is stabilising so more exchange leads to more stabilisation
Nitrogen has a higher IE relative to oxygen due to loss of exchange on ionisations
N -3K to -1K
O -3K to -3K
define electron attachment energy
the enthalpy change for adding an electron to an atom
What is the general trend for EAE in a periodic table
related to orbital energies so a lower orbital energy = larger Ea
Should decrease down a group
what are the exceptions for Ea in the periodic table
adding an electron to an element with a filled subshell is disfavoured Ea<0 - G2, G12, G18 going into an empty higher energy p or s orbital
O and F also has smaller than expected Ea
second period is different and 3rd period has highest Ea
Wy do O and F have different Ea
they have relatively small orbitals therefore adding an additional electron gives significant increase in electron Coulombic repulsion
why is the second period different for Ea
due to the small size of the 2p orbitals
what is Paulings definition of electronegativity
the ability of an atom to attract electron density towards itself in a molecule and correlates with lower valance orbital energies
describe the trend in electronegativity in the periodic table
generally increases across a period and decreases down a group due to increasing nuclear charge and decreasing atomic radius across a period
describe orbitals and energy
for energy when n is constant s<p<d<f
s orbitals penetrate better than p orbitals
describe orbitals and shielding
size of the orbital is related to the orbital type but mainly n
shielding is related to the number of angular nodes
s>p>d>f
what is meant by contracted first orbitals
1s 2p 3d 4f are the first of each type of orbital and so are not screened by orbitals of the same l quantum number so they are contracted in size and relatively low E
why do we have good covalent sigma and pi overlap
2p orbitals are small and close in size to 2s so good covalent bonding can occur due to high overlap
why does bond strength of M2 diatomics in the gas phase decrease
for higher n repulsion between the core electrons prevents close approach for high overlap integral so the bond is weaker
bond distance increases more rapidly than orbital size due to repulaion between core electrons so overlap decreases down a group
what is the general trend in orbital energies
an increase down a group groups 1 and 2 follow this trend but 13-17 have significant deviation from this trend especially in s orbital energies due to increasing nuclear charge and electron shielding effects.
which groups deviate from the trend due to allen electronegativity values
periods 4 and 6
describe why period 4 deviates from the trend in allen values
e.g. Gallium has a smaller covalent radius and is harder to ionise than aluminium which is an effect of the additional 10 e and 10 p from filling the d block
3d orbitals (2 angular nodes) are poor at shielding 4s and 4p electrons so they have higher electron probabilities near the nucleus than 3d so penetrate better
4p of Ga is similar in energy to 3p of Al leading to similar IE
what is the post transition effect
this is the general effect for the elements in period 4 that follow the transition series
due to the higher IE due to filling of the d block and decrease in E-X covalent bond strength down the group
describe how period 6 deviates from groups trends
4f orbitals shield poorly, both 6s and 6p orbitals experience increased Zeff
6s orbital is stabilised/contracted, and energy lower compared to that expected by the group trend
what are relativistic effects
for heavy elements the mass of electrons increase due to travelling at speed 50% of the speed of light and this effects 6s electrons the most
what is the inert pair effect
6s2 is inert and not used in bonding or ionisation
the most common OS for Tl +1 Pb+2 Bi+3
what two key factors affect the inert pair effect
variation in orbital energy and hence IE
trend is for covalent bond energies to decrease down a group so is difficult to compensate for IE
therefore TlCl3 is thermally unstable and decompose on heating due to lower oxidation state chlorides
TlCl3 —> TlCl +Cl2
How do ionic radii vary with oxidation state
The more positive the oxidation state the higher the Zeff is on the remaining electrons and therefore, the size is smaller
How might coordination number affect ionic radius
4 coordinate will appear smaller four molecules can be placed closer than 6 so 4 bond complexes are shorter and seem to be smaller
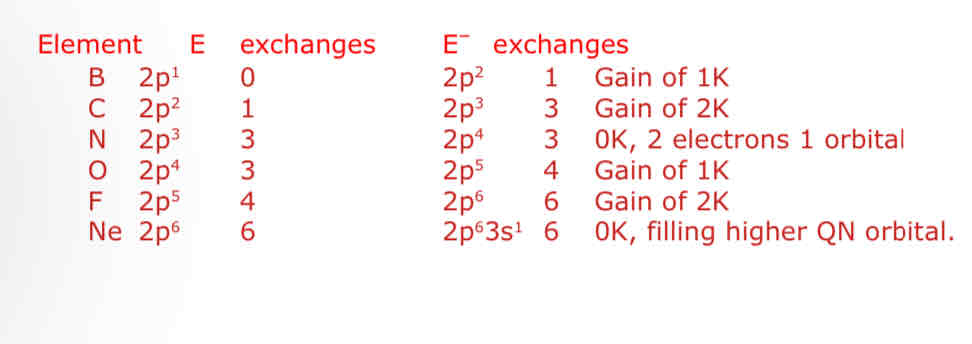
Account for the variation in electron attachment values across the 2nd period of the p block - B, C, N, O, F, Ne
how would electronegativity vary with oxidation state?
As oxidation state increases the orbital energy will become more negative and more stabilised therefore, electronegativity increases with oxidation state
Suggest reasons to explain why gallium and mercury are low melting point metals
Due to insertion of d-block and f-block elements, orbitals of the elements that follow are contracted leading to less good overlap and hence atoms are held less tightly
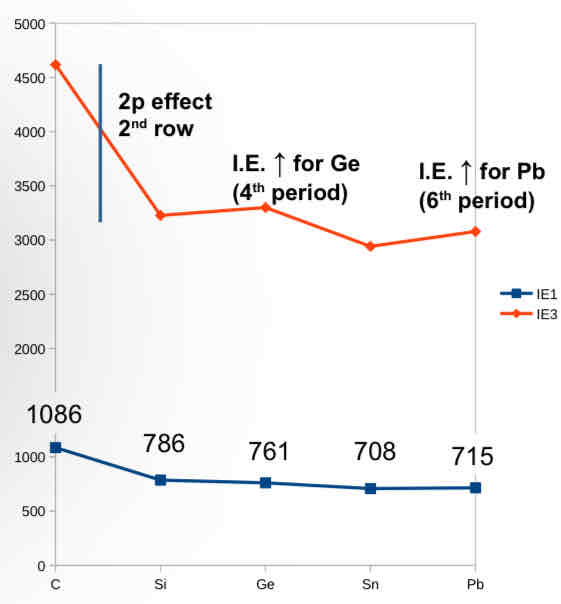
Past paper question -
Sing your knowledge of periodic trends briefly explain the variation in first and third ionisation energies for the group 14 elements
IE3>IE1 and there is a general trend of IE decreasing down a group
From period 2 to 3 there is a big decrease due to 2p second row effect. High Zeff of row 2 elements due to first filling of 2p
Post transition effect for period 4 added first 10 d block electrons in poorly shielding orbitals and 10 protons, so radius decreases and Zeff and IE increase
Post lanthanide and relativistic “inert pair effect” - for period 6 filled the f orbitals which shield poorly and relativistic effect leads to decreased radius and increased Zeff and IE
describe what the difference in electronegativity between atoms dictates
large difference indicates ionic bonding whereas a small difference indicates covalent or metallic bonding
describe what average electronegativity in a compound is related to
related to the directionality or localisation of the electron density
low average means little directionality, metallic bonding
high average means directional bonding, covalent molecules
describe the graphical representations of average electronegativity and difference in electronegativity
Van Arkels triangle
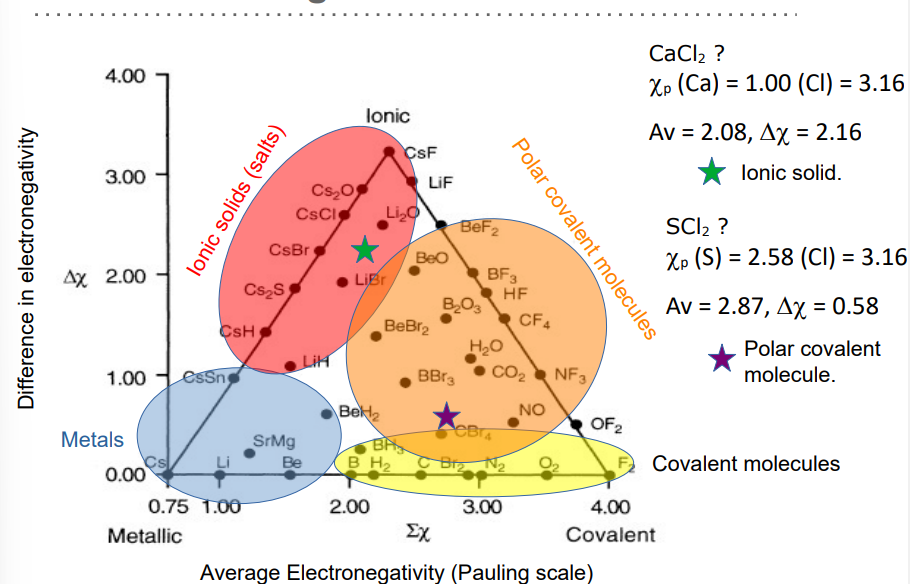
how can knowing bonding type allow us to predict key properties
large differences in orbital energies lead to ionic structures - 3D structures, high melting and boiling points, conduct electricity when molten but not when solid
small differences in orbital energies lead to polar covalent molecules- structures with discrete molecules, relatively low melting and boiling points and are unable to conduct electricity when molten or solid
what can lattice structures be described a
close packing of atoms or of ions with other ions occupying octahedral/ tetrahedral holes
what are the different types of lattice structures
Body centred cubic
face centred cubic CN-6
face centred cubic CN-4
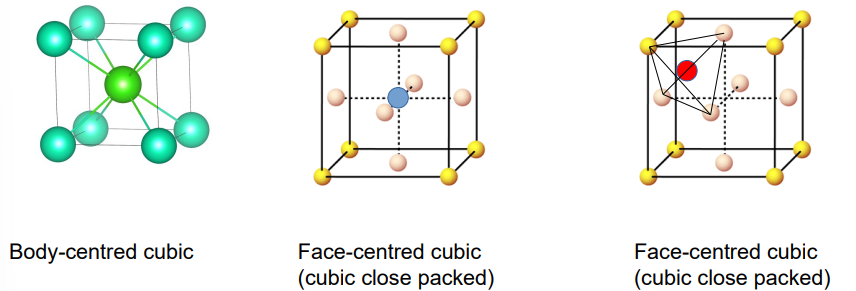
what are the 3 radius ratios
BCC - 0.732
FCC - (CN-6) - 0.414
FCC- (CN - 4) - 0.225
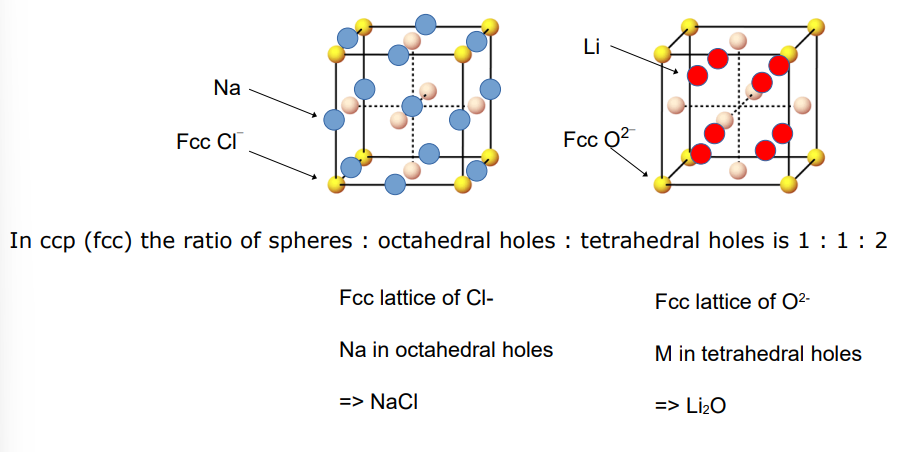
How do you derive the molecular ratio of atoms in a lattice
depends on whether they occupy the tetrahedral or octahedral holes
1:1 octahedral
1:2 tetrahedral
what are the assumptions when calculating lattice energy
assuming that bonding is purely ionic and cations and anions are undistorted spheres
how do you calculate lattice energy

why might calculated lattice energies differ from experimental lattice energies?
small highly charged cations are strongly polarizing (have a high charge density) these cations will strongly attract electron density from polarizable anions (large, low charge) that leads to some degree of covalency
describe the structure of metal oxides
they are highly ionic and have a large difference in orbital energies (or electronegativities) extended structure are predicted by the radius ratio
High CNs and high melting point
describe the structure and properties of metalloids
polar covalent 3D networks of chains and sheets
describe the structure and properties of non-metal oxides
covalent molecules, discrete with low CN and low melting points
describe the reactivity of highly ionic oxides
e.g. Na2O are Bronsted bases
the highly basic oxide deprotonates water.
And analogously for the hydroxides also act as bases

describe the reactivity trends for covalent oxides
on dissolution in water they are Bronsted bases. They form very stable anions as electronegative atoms stabilise negative charge due to low energy orbitals anf high energy of Attraction H2SO3—>HS03(-)—>SO3(2-)
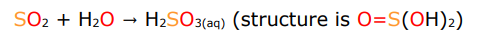
describe the synthesis of hydrogen
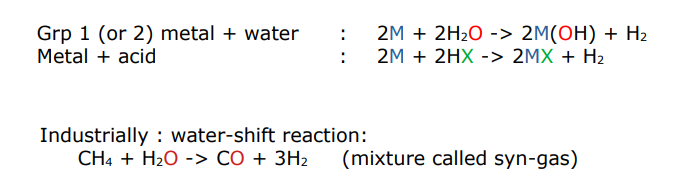
how do hydridic substances react?
they react as either a hydride ion(H-) donors of contain a d- hydrogen
what are the major types of hydrides
ionic hydrides and molecular hydrides
how are ionic hydrides formed
they are formed by heating group 1 or 2 metal with hydrogen

what are the general properties of ionic hydrides
predominantly ionic bonding occurs so it is a solid with a high melting point
they are highly reactive for example they react exothermically with protic sources such as water and Bronsted acids to form hydrogen
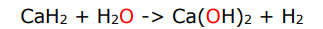
how are ionic hydride used in synthetic chemistry
they can be used as reducing agents
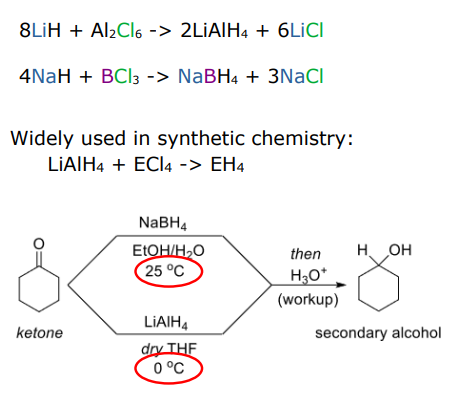
why is BH4(-) less reactive than AlH4(-)
B-H bond are stronger than Al-H
Al-H bond is more polarised because based on electronegativity H>B>Al
describe the properties of covalent hydrides
thermodynamic stability of hydrides decreases down a group and acidity decreases down a group
why does acidity decrease down a covalent hydride group
weaker E-H bonds due to poorer orbital overlap between 1s orbitals of H and the increasingly large np orbital so covalent bond strength decreases down the group
describe hydrogen bonding
formed between a hydrogen attached to an electronegative atom and an electronegative element with a lone pair of electrons and is responsible for the melting and boiling points of p-block molecular orbitals
describe the range of bond polarities in covalent hydrides
the range of bond polarities depends on orbital energy (electronegativity)
acidic - polar H-X and hydrogen has a significant dipolar (+) charge HCl
weakly protic (hydrogen has a smaller dipole(+)) such as CH4(+)
hydridic character (hydrogen with a negative dipolar charge) such as SiH4
What is the radius ratio of a BCC
Over 0.732
What is the radius ratio of a face centred cubic with CN 6
0.414-0.732
What is the radius ratio for face centred cubic with CN 4
0.225-0.414
What are the general properties of s-block metals
Soft reactive metals with smooth trends in most properties
Low electronegativity so the valence orbitals are high in energy
Bonding is predominantly ionic
Widely occurring in nature but not as the metal salts
How are s-block metals isolated
Isolated by electrolysis
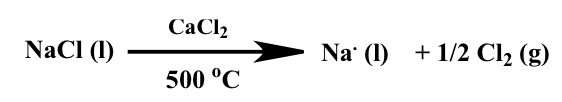
How do we write and represent electrolysis
It is written as two parts, an oxidation and reduction half-reaction
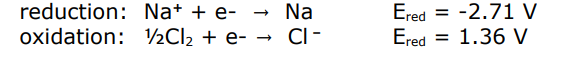
what equation describes if the oxidation and reduction in electrolysis are spontaneous
if E is negative the the Gibbs energy will be positive so its not spontaneous
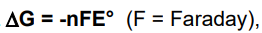
how do you know if you have a strong oxidant or reductant
the more positive a reduction potential the stronger the oxidant
the more negative the reduction potential the stronger the reductant
describe the properties of group 1 metals
alkali metals are all strong reducing agents with heavier elements being stronger reducing agents
(except for lithium as it has the most reducing E potential)
describe the group 1 reactions with hydrogen
they react to make ionic hydrides
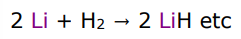
describe group 1 reactions with nitrogen
they react to give ionic nitrides (N3-)
describe group 1 reactions with oxygen
they react with excess oxygen but the product depends on the metal due to greater stabilisation for larger anions with larger cations
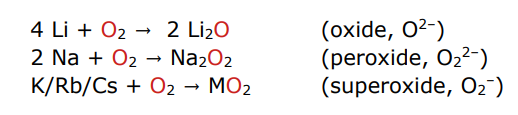
describe salt dissolution for most group 1 metals
dissolution and solubility is a balance between lattice and solvation energies. For dissolution to be spontaneous the change in Gibbs free energy must be negative so the change in entropy must be large and positive
Dissolution is favoured if:
DGsolvation > DGlattice
what is lattice energy proportional to?

what is the energy profile of dissolution
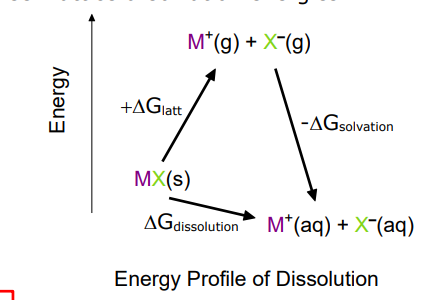
describe what occurs during dissolution
lone pairs of solvent molecules act as a lewis base and coordinate to the metal ion
in the s and p block there is no ligand field stabilisation and the hydration energy is controlled by the size and charge on the metal cation
why is lithium different when it comes to salt dissolution
lithium has the smallest primary hydration sphere but the largest secondary hydration sphere as it has the highest charge density
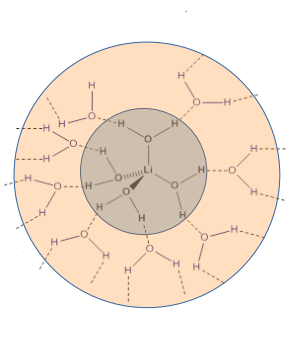
why does Lithium have the most reducing E value
This is due to the greater solvation energy overcoming the higher ionisation energy compared to other group 1 metals
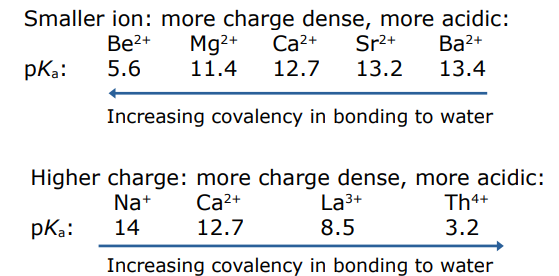
describe the Bronsted acidity of a group group 1 metal hydroxide
If the metal is charge dense it can polarise the metal hydroxide bond weakening the O-H bonds in water so the dissociation of the H+ occurs to ta greater extent making the solution more acidic
smaller ion: more charge dense, more acidic
higher charge: more charge dense more acidic
what is the Ka equation
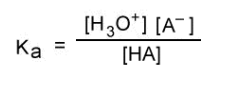
what cavity diameter of pre-organised ligand is optimal for Li+
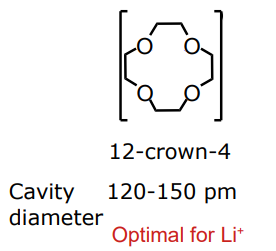
what cavity diameter of pre-organised ligand is optimal for Na+
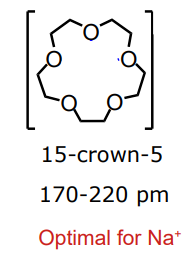
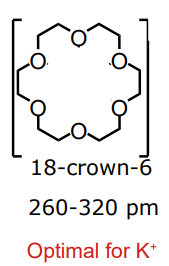
what cavity diameter is optimal for K+ from a pre-organised ligand
describe the properties of group 2 metals
theyre smaller than group 1 and virtually always lose both electrons: +2 ox state
Major contraction on formation of the M2+
exceptions are Be and Ra
what compound are group 2 metals naturally found as
carbonates of sulphates
will group 2 salts generally be more or less soluble than group 1
lattice energy is proportional to z+z-/r
group 2 metal ions are smaller and higher charge than group 1 so group 2 salts have larger lattice enthalpies than group 1 therefore they are less soluble
both carbonates and sulphates decompose on heating, will they form oxides, superoxides or peroxides
Group 2 ions are smaller than group 1 so we would expect the smallest anion, ie the oxide
will the 1st IE for Ra be higher or lower than Ba
It will be higher due to relativistic effects
describe the anomalous Beryllium in group 2
its extremely small and has the CN 4
High charge density so it is highly polarizing with significant covalent bonding
salts are very acidic in water
beryllium hydroxide is amphoteric
Beryllium and its compounds are distinctly different from other group 2 elements
why is Hg different from other group 12 elements in terms of ionisation energy
relativistic effects
define chalcophilic
naturally occuring as sulfides
how do we form group 1 organometallic compounds
made via metal + halocarbon