Percentage yields
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
Why would you sometimes not get the maximum expected amount of product?
The reaction may be reversible (both the forwards and backwards reaction can take place)
Some of the product may be lost when it’s separated from the reaction mixture
Some of the reactants may react in other reactions
2
New cards
Equation to calculate % yield

3
New cards
Example of question
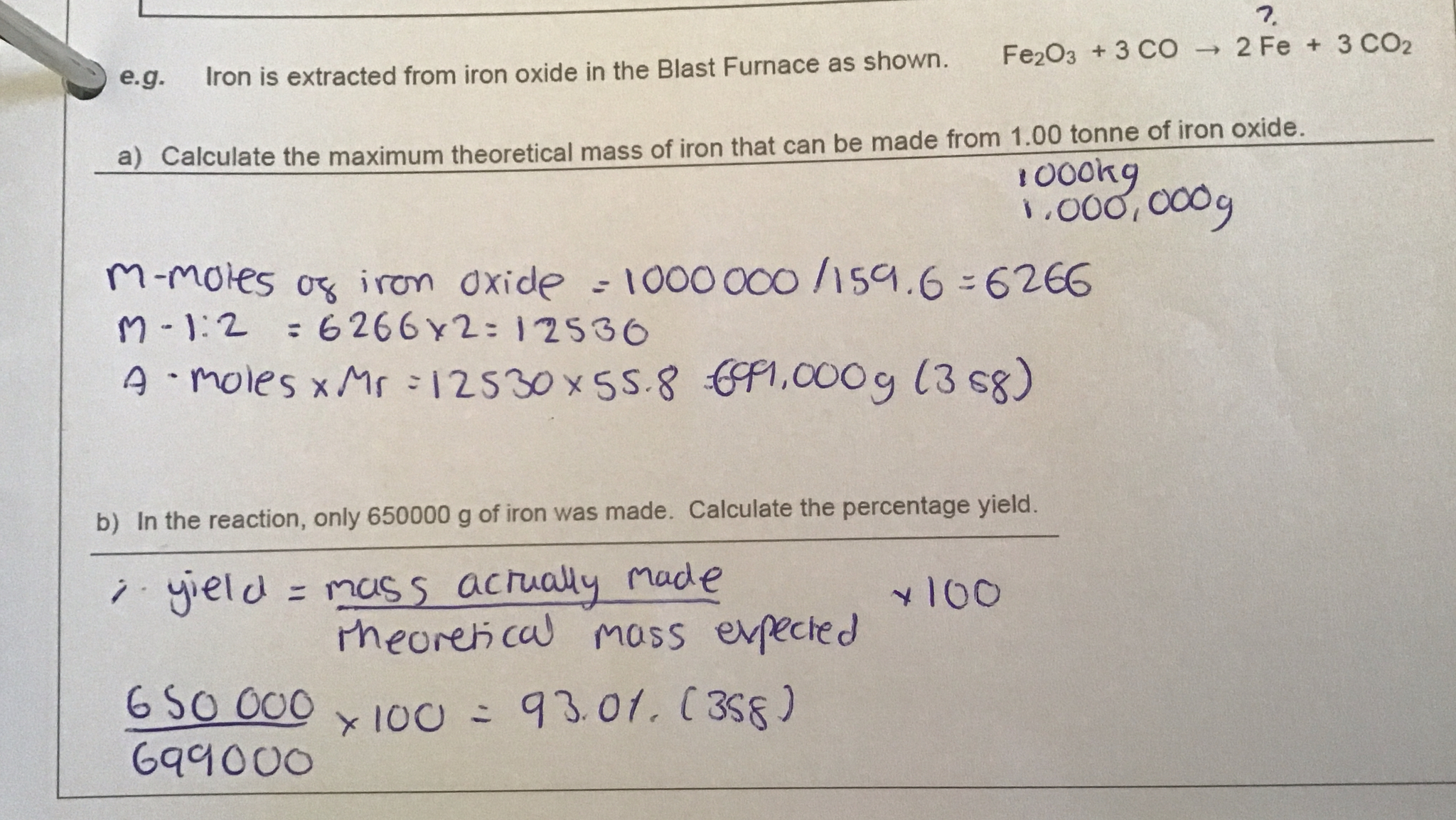
4
New cards
.