Reservoir Final
1/70
Earn XP
Description and Tags
Modules 9-12
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
71 Terms
What are the P and T conditions for the Ideal Gas Law?
Low P
High T
What are the assumptions of the Ideal Gas Law?
No interactions between gas molecules (low P)
No volume occupied by gas molecules
An instrument that uses the Ideal Gas Law to measure effective porosity of core plug samples
Helium Porosimeter
What does a Helium Porosimeter measure?
Grain Volume
Effective pore volume can then be calculated from:
PVe = BV - GV
What is the Real Gas Law?
A modified EOS (ie. relates V to P&T) that can be used for gases at any T & P.
Z (Compressibility Factor) corrects for volume changes due to changes in T & P
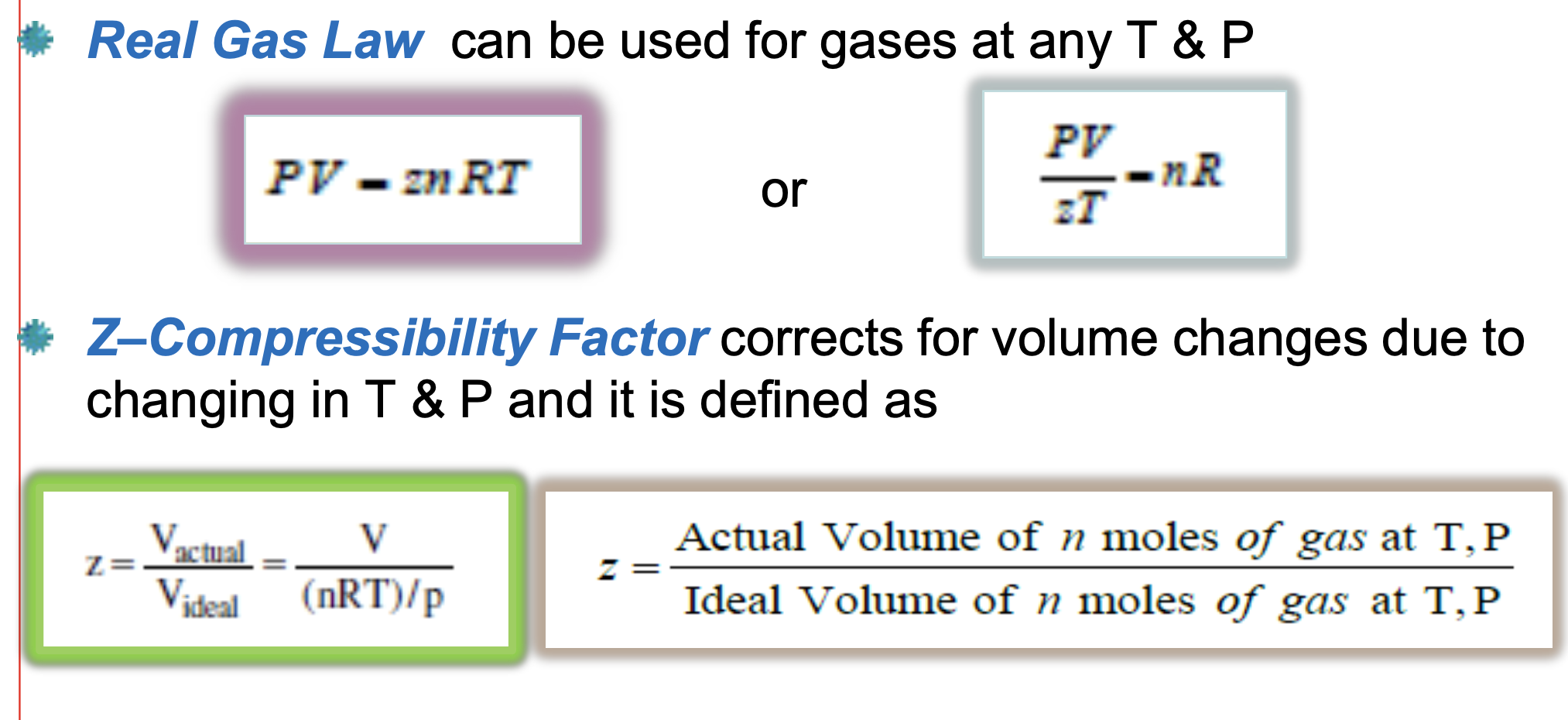
Why is the Z-factor different for different gases?
Due to the varying Tc & Pc of the different gases
The Law of Corresponding States says that the Z-Factor of all pure gases depends only on the gas' ___________ and ___________.
Reduced Temperature (Tr)
Reduced Pressure (Pr)
A gas mixture that is assumed as one component
Pseudo Gas
Because of Avogadro’s Law, what can be assumed about the Volume Fraction of a given component of a gas mixture and its Mole Fraction?
That they are equal.
ie. Volume Fraction of component i = Mole Fraction of component i
Avogadro’s Law: equal volumes of gases, at the same temperature and pressure, contain an equal number of molecules.
What are Pseudo-Critical Properties?
Used to treat a gas mixture as if it is a single component gas
Essentially a weighted sum of each component’s Tc and Pc
For a pure gas, Z-Factor is a function of ___ and ___.
Similarly, for a gas mixture, the Z-Factor is a function of ___ and ____.
Pure gas → Tr and Pr
Gas mixture → Tpr and Ppr
When calculating pseudo-critical P and T, what units must be used for all T & P values?
T → K
P → kPaa
What are the most common non-hydrocarbon reservoir gases?
N2
CO2
H2S
What is acid gas?
A gas mixture containing significant amounts of acidic gases:
N2
CO2
H2S
Which gases cause a deviation of pseudo-critical properties in a mixture, and hence must be adjusted for?
H2S
CO2
Use chart or ε formula
What are Corrected Pseudo-Critical Properties?
Used when treating a mixture containing H2S and/or CO2 as a single component gas
T’pc
P’pc
What is the Gas Formation Volume Factor ?
Bg
The ratio of the volume of a given mass of gas at reservoir conditions to its volume at standard conditions

What is the Gas Formation Expansion Factor (Eg) ?
The reciprocal of Bg
What are the units for Bg and Eg ?
Bg → rm3/sm3
Eg → sm3/rm3
What are the assumptions made for Closed Tank Gas Reservoirs?
Reservoir contains only Gas Phase + Water Phase
Water phase is at Swirr
Zero gas dissolved in formation water
Reservoir Pressure is not maintained during production lifetime
Reservoir is isolated from aquifers/other formations
Reservoir gas composition remains constant during production
Reservoir Temperature stays constant during production
Compressibility of Reservoir Rock is negligible
Compressibility of Formation Water is negligible
If the volume of gas at surface conditions is known, how would you calculate the volume of gas at reservoir conditions?
Multiply it by Bg
∴ IGIP or G (initial resource @ STP) can be multiplied by Bgi to get the IGIP @ initial reservoir T & P
From the Gas Material Balance Equation, define:
Gp
P
Bg
G-Gp
(G-Gp)Bg
Gp → Volume of Cumulative Gas Production @ STP
(se6m3 or MMSCF)
P → Current Reservoir Pressure
ie. the P that is declined from Pi after producing Gp
Bg → Gas FVF @ reservoir P & T
G-Gp → Remaining gas Volume in reservoir @ STP
(se6m3 or MMSCF) (ie. after producing Gp)
(G-Gp)Bg → Remaining gas Volume in reservoir @ reservoir P & T
(se6m3 or MMSCF) (ie. after producing Gp)
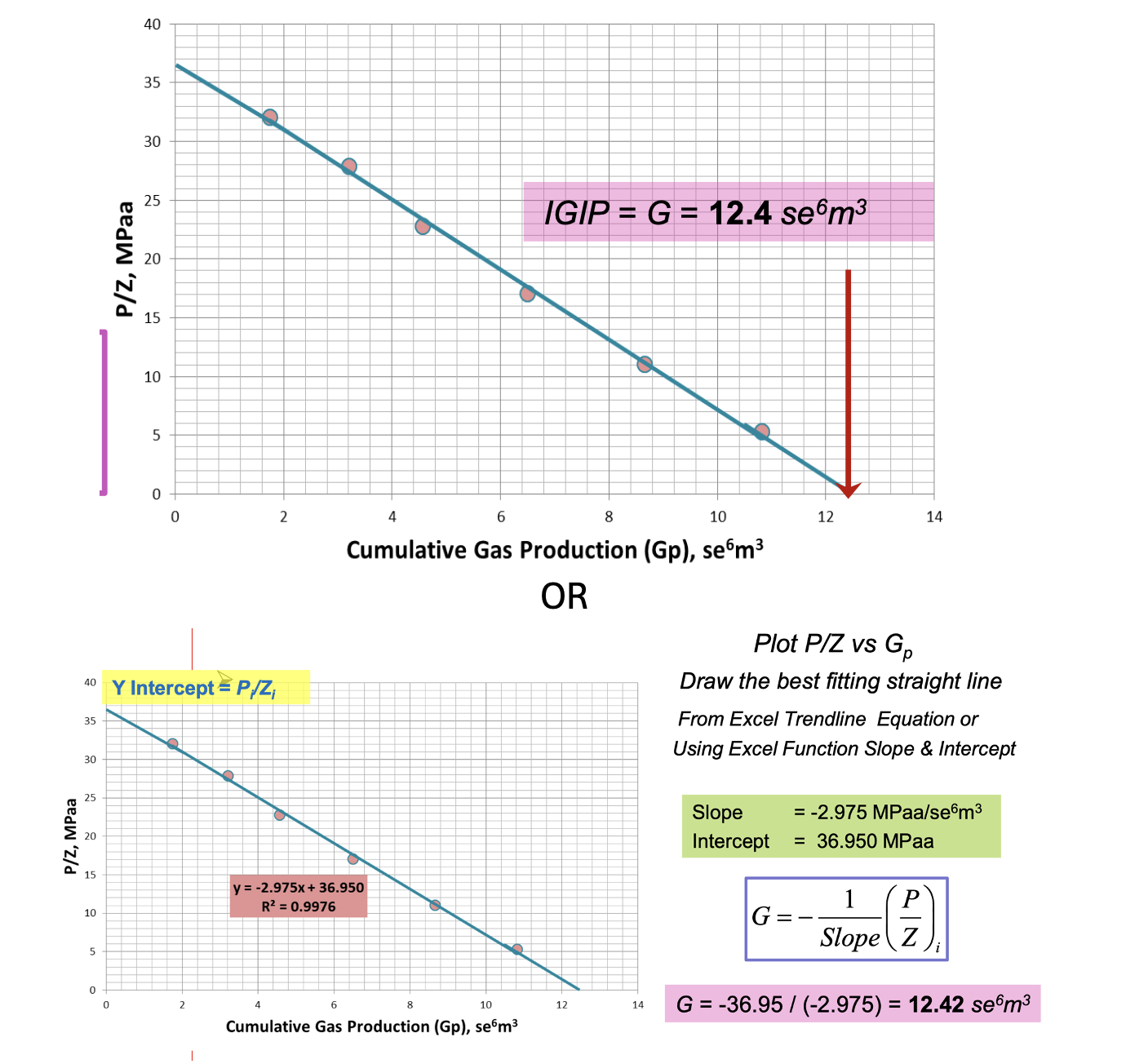
What are the 2 methods of determining IGIP, using material balance?
Using P/Z vs. Cumulative Gas Production (Gp) Graph
y-intercept = (P/Z)i
x-intercept = IGIP = G (ie. Gp @ P/Z = 0)
Using P/Z Equation
IGIP = G = (-1/slope)(P/Z)i
In other words you are solving for the x-intercept ie. the x-coordinate (Gp) when the y-coordinate (P/Z) equals 0.
∴ you’re using y = mx+b to find x when y = 0
ie. finding Gp in (P/Z)i = mGp + (P/Z) when (P/Z) = 0
When is a gas reservoir at its economic limit?
When:
Cost of Producing Gas = Total Income
What is the Abandonment Production Rate (GRa) ?
The rate of gas production at the economic limit
se3m3/day
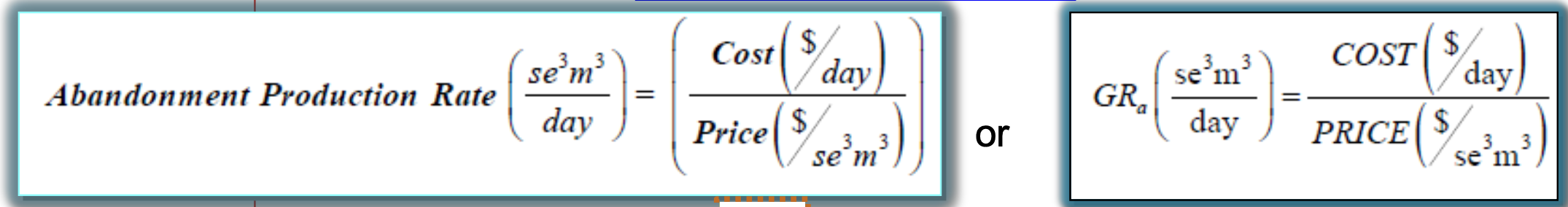
What is the relationship between the abandonment rate and the cost of production?
What is the relationship between the abandonment rate and the gas price?
Gas Price ↑ , Abandonment Rate ↓
ie. if gas prices are high, you can produce less to break even
Cost ↑ , Abandonment Rate ↑
ie. if your costs of production are high, you need a high production rate to break even
NOTE: these relationships can be derived from the formula for calculating GRa on the formula sheet
What is another way of defining Initial Reserves?
Initial Reserve = Gpa = Recoverable Gas
ie: The initial reserve is equal to the cumulative production at abandonment conditions (se6m3)
this is because reserves are defined as the volume of gas that can be produced economically
Define these various gas volumes:
Gpa
Gps
Gr
Gpar
Gu
Gpa → Cum. gas prod. @ abandonment = recoverable gas = initial reserve (se6m3)
Gps → cum. gas production to date (se6m3)
Gr → Remaining gas in place = remaining resource (se6m3)
Gpar → Remaining cum. gas production = remaining reserve (se6m3)
Gu → Unrecoverable gas (se6m3)
What are the assumptions for the Oil Material Balance (Stock Tank Oil Reservoirs) ?
The Oil Reservoir:
is @ initial conditions (not yet in production)
contains only oil phase + water phase (@Swirr)
pressure is not maintained during production lifetime
is not connected to any water aquifer
no gas/water injection
temperature remains constant during production
compressibility of reservoir rock is negligible
compressibility of formation water is negligible
no gas dissolved in reservoir water
When is an oil reservoir considered to be undersaturated?
When Pres ≥ Pb
ie: when the reservoir pressure is greater than or equal to the bubble point pressure
all available gas is dissolved in the oil (ie. no free gas)
For Oil Reservoir Material Balance, define:
N
Boi
NBoi
Np
Pi
P
Bo
N-Np
(N-Np)Bo
N → IOIP = Initial Resource @ STP (sm3)
Boi → Initial Oil Formation Volume Factor (rm3/sm3)
NBoi → IOIP @ initial reservoir T&P (ie. Ti & Pi) (rm3)
Np → Cumulative oil production @ STP (sm3)
Pi → Initial reservoir pressure (kPaa)
P → Current reservoir pressure (ie. after producing Np) (kPaa)
Bo → Oil FVF @ current reservoir P&T (rm3/sm3)
N-Np → Remaining oil volume in reservoir = remaining resource @ STP (sm3)
(N-Np)Bo → Remaining oil volume in reservoir = remaining resource @ reservoir P&T (rm3)
If the volume of oil at surface conditions is known, how would you calculate the volume of oil at reservoir conditions?
Or in other words, how would you calculate the Live Oil Volume if the Dead Oil Volume is known?
Multiply it by Bo
∴ IOIP or N (initial resource @ STP) can be multiplied by Boi to get the IOIP @ initial reservoir T & P
For Oil Reservoir Material Balance, when Pres< Pb, define:
Rp
RF
Rso
Rsoi
Bg
Rp → Production GOR (sm3/sm3)
RF → Recovery factor = Np/N
Rso → Dissolved (solution) GOR @ current reservoir P&T (sm3/sm3)
Rsoi → Initial dissolved (solution) GOR (sm3/sm3)
Bg → Gas FVF @ current reservoir P&T (rm3/sm3)
Recall: Rso stands for Ratio Solution Oil (ie. Solution GOR)
What is the difference between FVFs (ie. Bo and Bg) and the various GORs (ie. Rso, Rp, Rsoi) ?
Formation volume factors (FVF) are used to adjust for the difference in volume of a single phase of oil (Bo) or gas (Bg) when at reservoir conditions and at surface conditions.
Gas to oil ratios are used to define the ratio of a gas volume to an oil volume at various conditions. ie:
At surface: Rp (production GOR)
At reservoir: Rso (dissolved GOR)
At reservoir initially: Rsoi (initial dissolved GOR)
For GORs, under which conditions (surface or reservoir) are they all measured in?
Surface conditions (all will have units sm3/sm3)
ie:
Production GOR (Rp) → The actual measured GOR at the surface (sm3/sm3)
Dissolved (solution) GOR (Rso) → the amount of gas that would come out of solution if the oil is brought to surface over the amount of oil at surface (sm3/sm3)
∴ GORs will always have surface units
Under what conditions does Rp = Rso ?
Only in an undersaturated reservoir.
This would mean that no free gas is present, and hence no free gas is produced, so therefore Rp must equal Rso.
Under what conditions is Rp > Rso ?
When Pres < Pb
this would indicate the presence of free gas in the reservoir, which will be produced along with the gas dissolved in the oil, leading to an Rp that is greater than the Rso.
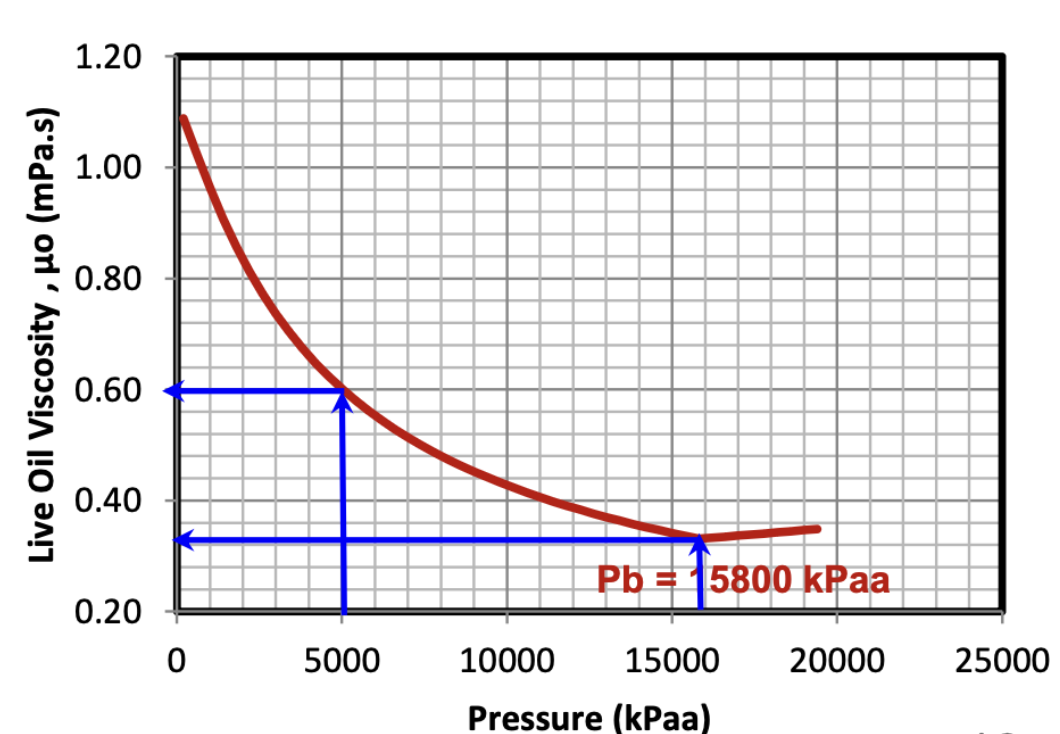
When Pres < Pb, what happens to the live oil viscosity as the reservoir pressure declines?
It increases
as pressure declines while below the Pb, dissolved gas comes out of solution, and the oil loses it’s “lightening agent”, hence the viscosity of the oil increases.
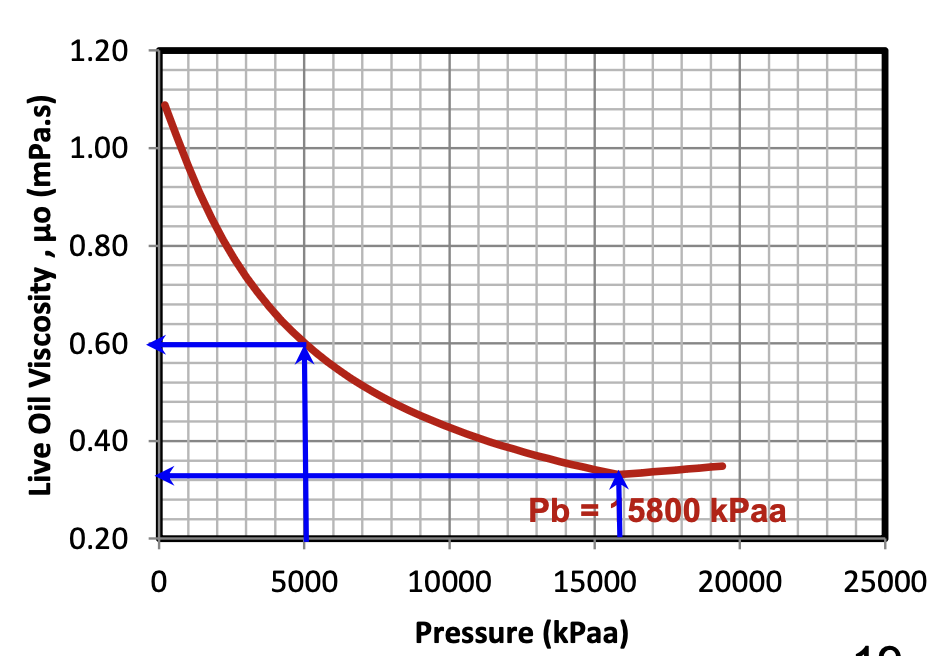
When Pres > Pb, what happens to the live oil viscosity as the reservoir pressure increases?
It increases slightly
All of the available gas remains dissolved in the oil above the Pb, but as the pressure increases, the oil itself will compress slightly, leading to a slight increase in the oil’s viscosity
What are the assumptions used in Darcy’s Law?
Oil/Water are incompressible
Single phase
pores contain 100% oil or water - no gas
Horizontal flow (no gravity effect)
Laminar flow
Steady-state flow (doesn’t change over time)
Fluid doesn’t react with the rock
A medium with a permeability of 1 darcy permits a flow of ____ cm³/s of a fluid with viscosity of ____cp under a pressure gradient of ____ atm/cm acting across an area of ____cm².
A medium with a permeability of 1 darcy permits a flow of 1 cm³/s of a fluid with viscosity of 1 cp under a pressure gradient of 1 atm/cm acting across an area of 1 cm²
Under what conditions is k equal to the absolute permeability?
When only a single phase fluid is present
Under what conditions is k equal to the effective permeability (ke)
When two or more fluids are present
TorF: The absolute permeability of a rock is constant and independent of fluid type flowing through it
True
TorF: Absolute permeability is independent of the pressure gradient
True
An ideal reservoir wherein the reservoir properties (e.g. k) are assumed to be the same everywhere
Homogeneous Reservoir
An actual reservoir with variation of reservoir properties laterally and aerially (vertically)
Heterogeneous Reservoir
In a vertically fractured rock, which would be greater: kv or kh ?
kv > kh
What is k90?
The horizontal permeability at a 90° angle from the maximum permeability direction
Which logs are used to measure porosity?
Neutron density log
Acoustic log
How is permeability typically measured empirically?
In a lab using a core sample
The relationship between porosity and permeability
log(k) = b+ (mΦ)
is most likely to be accurate in which of the following:
A) carbonate formations
B) fractured reservoirs
C) sandstone formations
C) sandstone formations
What are the 2 horizontal flow patterns in reservoirs?
Linear
Radial
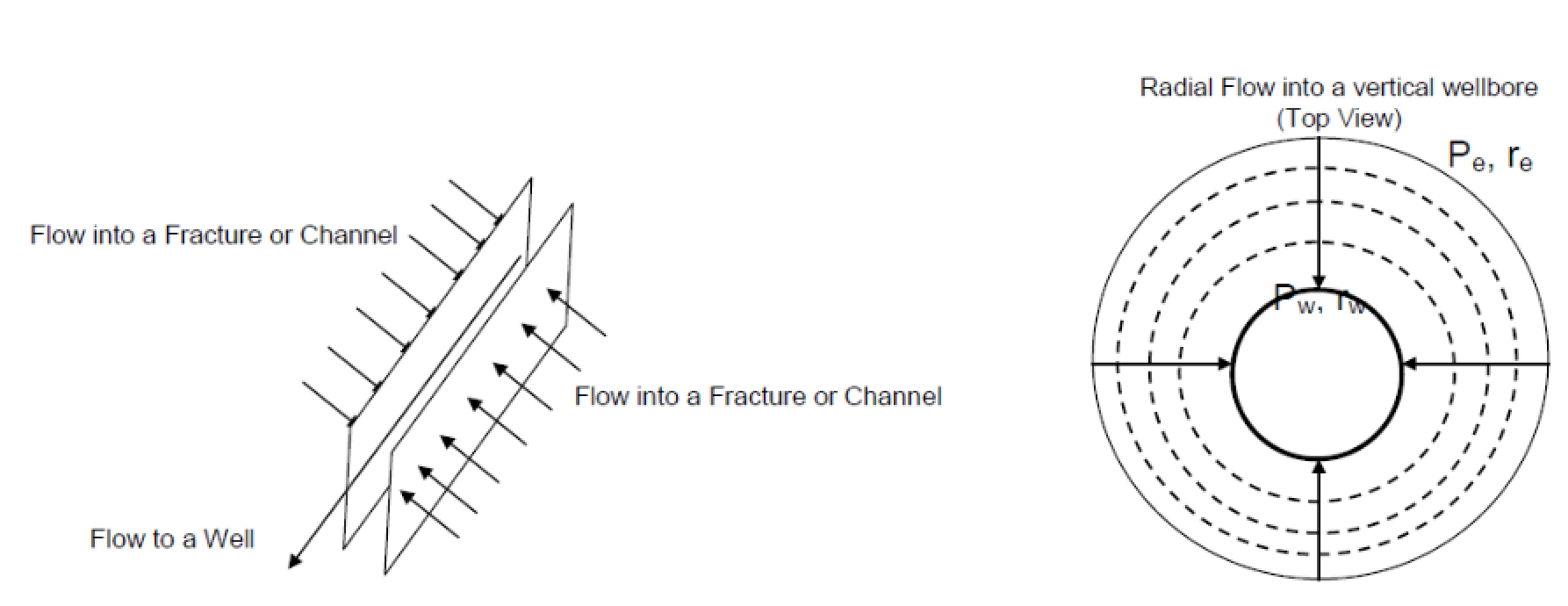
The boundary of the region where fluid flows to a well
Drainage radius (or external radius or effective radius) re
The area inside the drainage radius
Drainage area
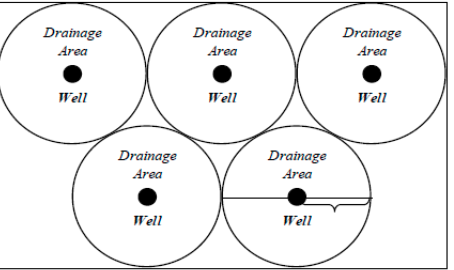
How is the drainage radius (re) calculated?
½ of the distance between 2 consecutive wells
ie. re = ½ x (distance to nearest well)
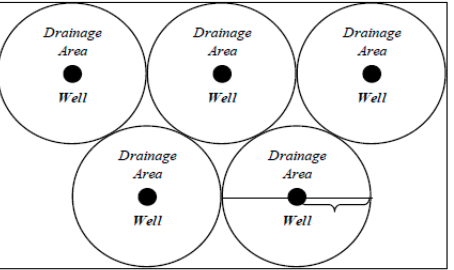
The ratio of the total area of the field to the number of producing wells
Well Spacing (Aw)
normally reported in acres or hectares per well

What are the 2 methods that can be used to calculate the drainage radius when the well spacing is known?
Circle inside square model (on formula sheet)
π Model
can be derived from Aw = πre²
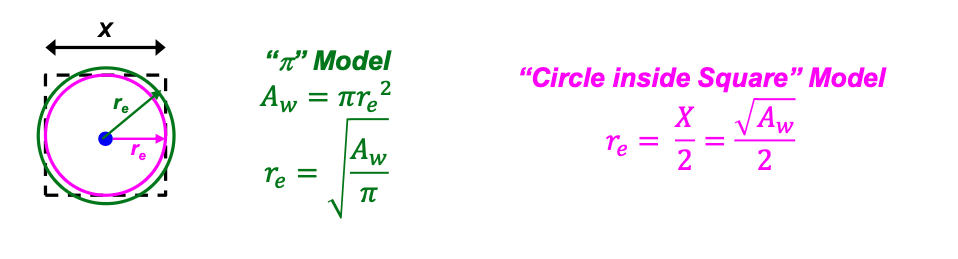
Which will be larger: the re calculated using the π Model or the re calculated using the ‘circle inside square model’ ?
The re from the π Model
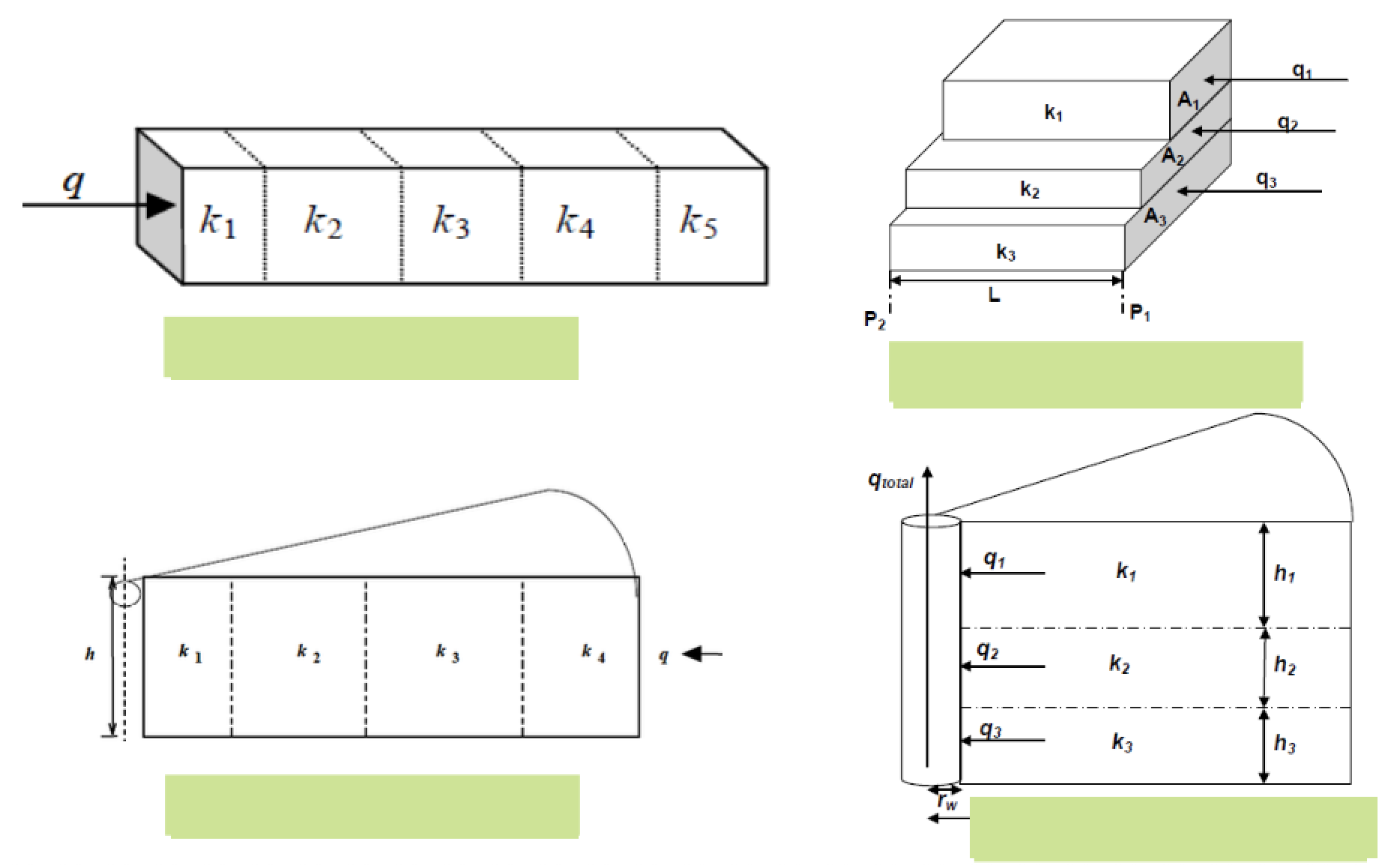
Label each type of multi-layer flow
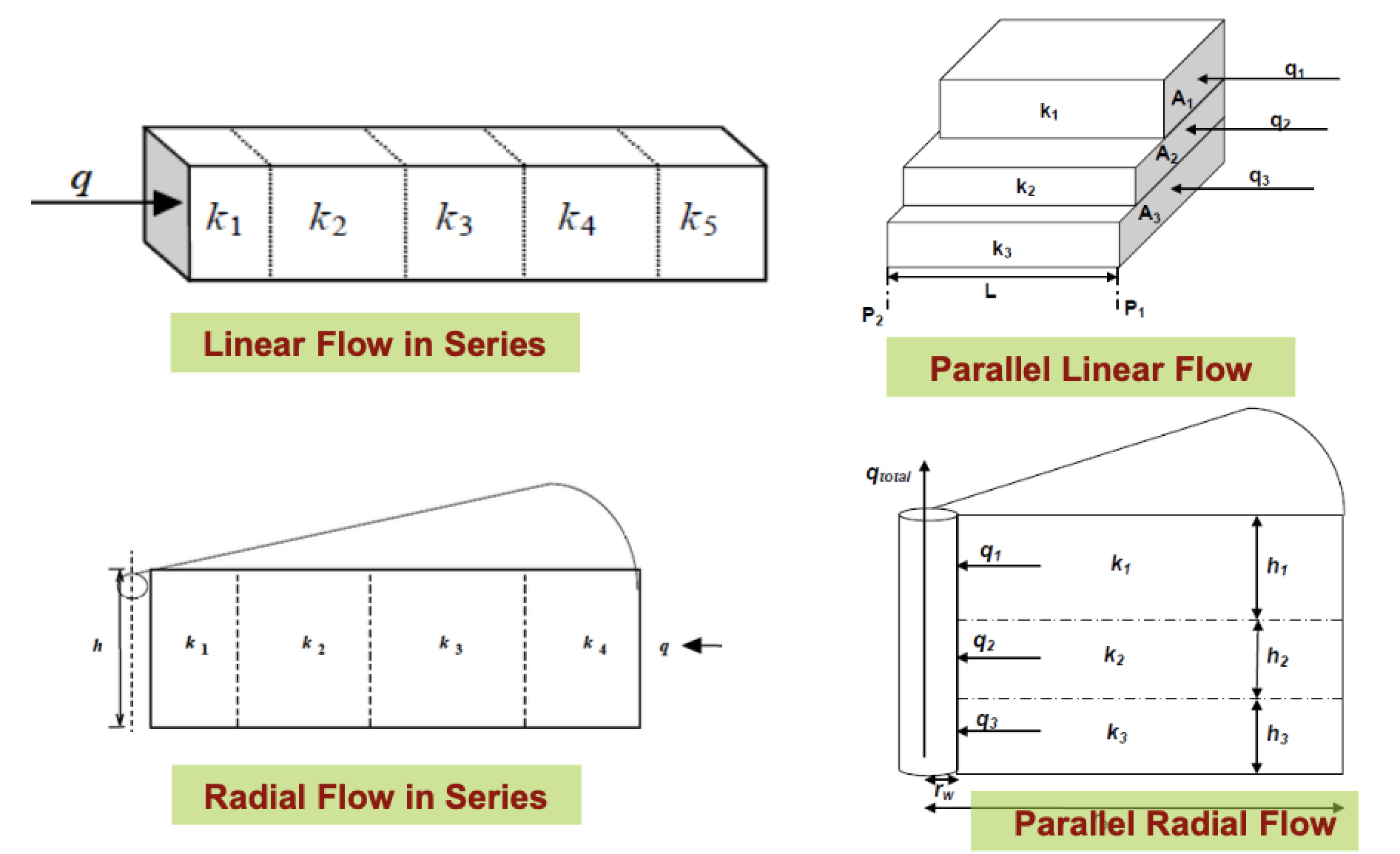
Which equations would you use to calculate the average porosity and the zonal flow rates in a stratified formation?
Stratified = parallel
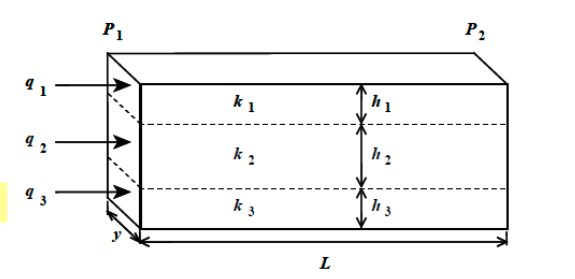
The point at which a gas and its liquid phase become indistinguishable
Critical point
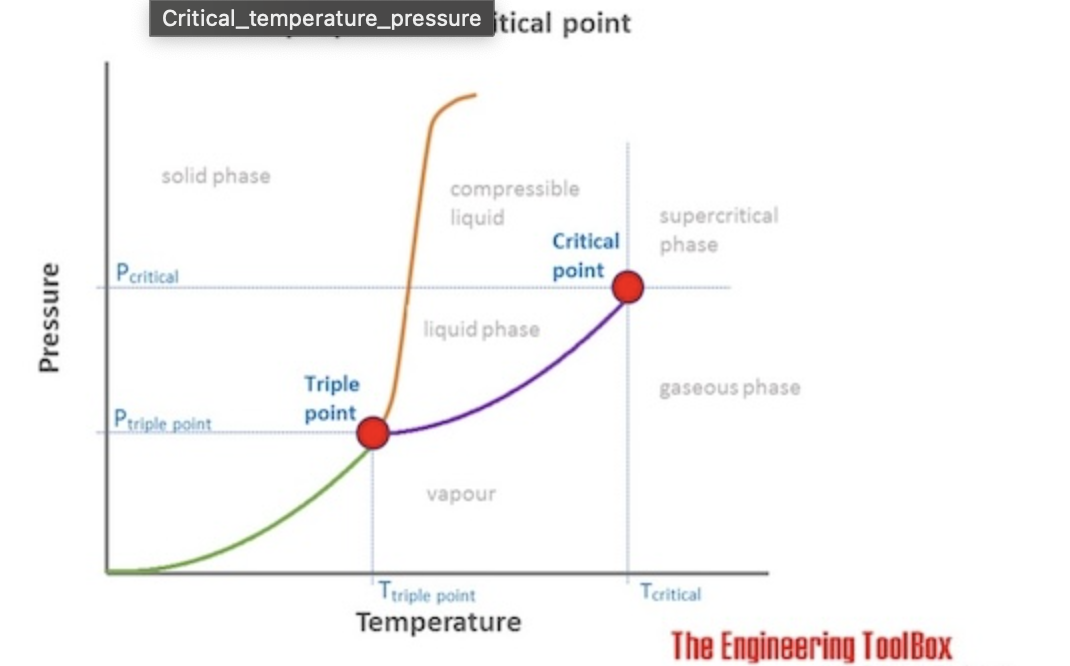
The temperature above which a gas cannot be liquefied by pressure alone
Critical Temperature Tc
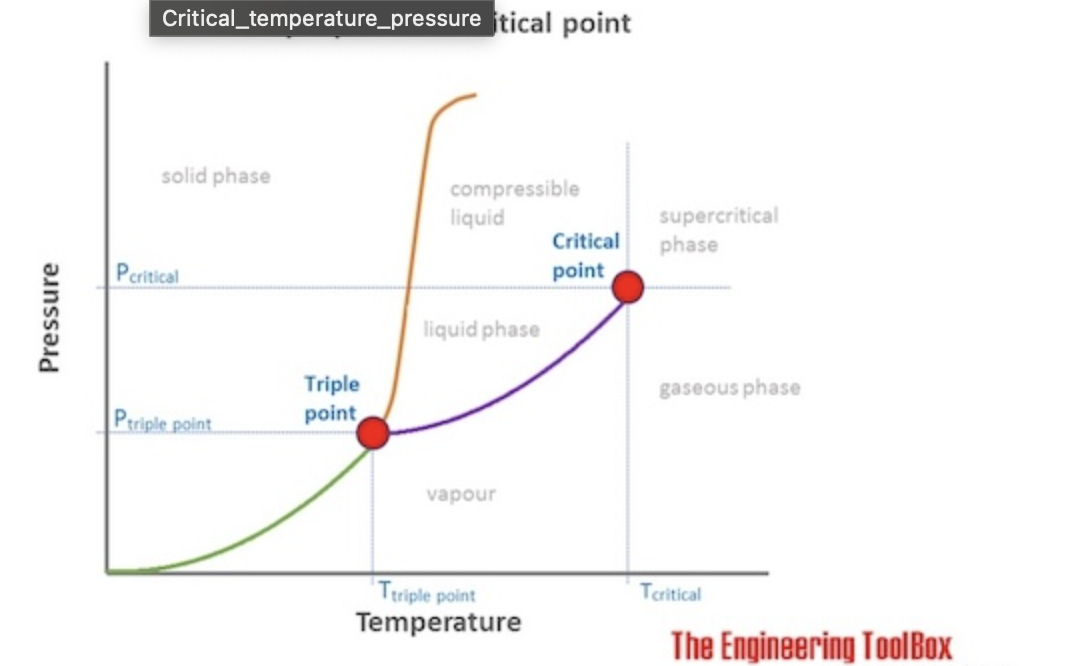
The minimum pressure required to liquefy a gas at its critical temperature
Critical Pressure Pc
What is another name for the gas compressibility factor (Z)?
Gas deviation factor
How would you calculate the volume of intial dissolved gas?
N x Rsoi
How would you calculate the volume of gas produced at surface?
Np x Rp

What must the units always be of a FVF, whether for oil or gas?
reservoir unit/surface unit
ie:
rm3/sm3
bbl/STB
What must the units always be of a GOR, whether it’s a solution/dissolved GOR (Rso) or a production GOR (Rp) ?
surface units/surface units
ie:
sm3/sm3
SCF/STB
What is the unit for permeability (k) ?
mD
Is the relationship between permeability and porosity defined as log(k) = b+ (mΦ) valid for fractured reservoirs?
No