Module 6 - Acid/Base Reactions
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
45 Terms
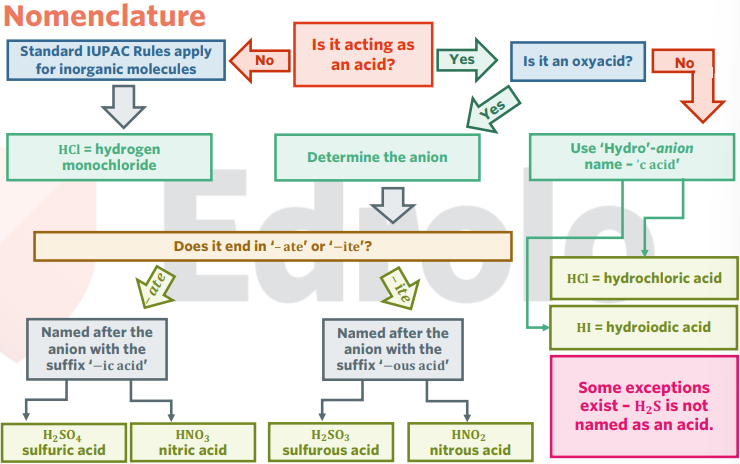
nomenclature
indicators
indicators are weak acid dyes that exist in equilibrium
HIn(aq) <-> H+(aq) + In-(aq)
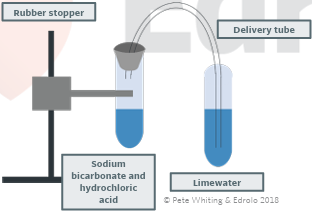
predict the products of acid reactions and write balanced equations to represent acid/carbonate reactions
metal carbonate + acid → carbon dioxide gas + salt + water
strength of the reaction depends upon the strength an concentration of the acid
exothermic
presence of carbon dioxide can be confirmed with the limewater test
hydrogen carbonates have the same products
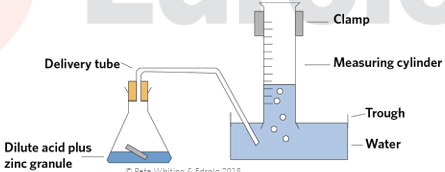
predict the products of acid reactions and write balanced equations to represent acid/metal reactions
metal + acid → hydrogen gas + salt + energy
strength of the reaction depends upon the strength an concentration of the acid and the position of metals on the activity series
exothermic — if it’s too exothermic (as in the case of group 1 metals), the H gas produced can explode
presence of H gas confirmed with the pop test
applications of neutralisation reactions in everyday life
ant bites
these are formic acid
they can be neutralised with a weak base
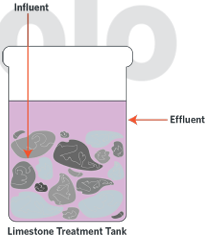
applications of neutralisation reactions in industrial processes
Industrial neutralisation of acids and bases requires proton transfer, this is exothermic.
On a large scale this can release large amounts of energy.
This can cause the water in the system to boil, spit, bubble and lift corrosive chemicals up as it bubbles and spits water droplets.
Chemicals can also be neutralised when spills/accidents occur.
An acid or base that is neutralised turns largely to harmless salts and water – but this reaction is exothermic so caution is necessary.
Lime stone (CaCO3) – a basic salt – can be used to neutralise acidic waste
enthalpy of neutralisation
neautralisation is a transfer of protons and proton transfer is always an exothermic process
apply standard enthalpy formula
include the mass of both solutions combined AND the moles of water produced
calculation steps
write the equation
calculate nwater
calculate the heat of neautralisation
calculate the enthalpy of neutralisation

Lavoisier to Davy theories
1776 - Lavoisier was the first to define an acid— as containing oxygen
1810 - Davy discovers various acids that do not contain oxygen, but they all contain hydrogen
Arrhenius’ theory
1884
acids ionise in water to give H+ which will bond to water and create the hydronium ion
bases ionise in water to give OH-
acid + base → salt + water
limitations
only applies to aqueous solutions
only applies to substances that give off those ions
Brønsted–Lowry theory
1923
an acid is a species that donates a proton to a base
a base is a species that accepts a proton from an acid
acid-base reactions involve proton transfer
overcame from Arrhenius:
not necessary to be in aqueous solutions
allows for amphiprotic substances (can act as an acid or a base)
limitations
still requires an acid to have a H+ to donate
pH formula
pH = -log[H+]
pH = 14 - pOH
calculation of pOH
pOH = -log[OH-]
pOH = 14 - pH
calculation of hydrogen ion concentration [H+]
write acid dissociation equation to find mole ratio of acid to H+ ions
find concentration of acid
therefore, find concentration of H+ ions
calculation of hydroxide ion concentration [OH-]
write base dissociation equation to find mole ratio of acid to OH- ions
find concentration of base
therefore, find concentration of OH- ions
write ionic equations to represent the dissociation of acids and bases in water
HA ⇌ H+ + A-
HB ⇌ OH- + B+
conjugate acid/base pairs
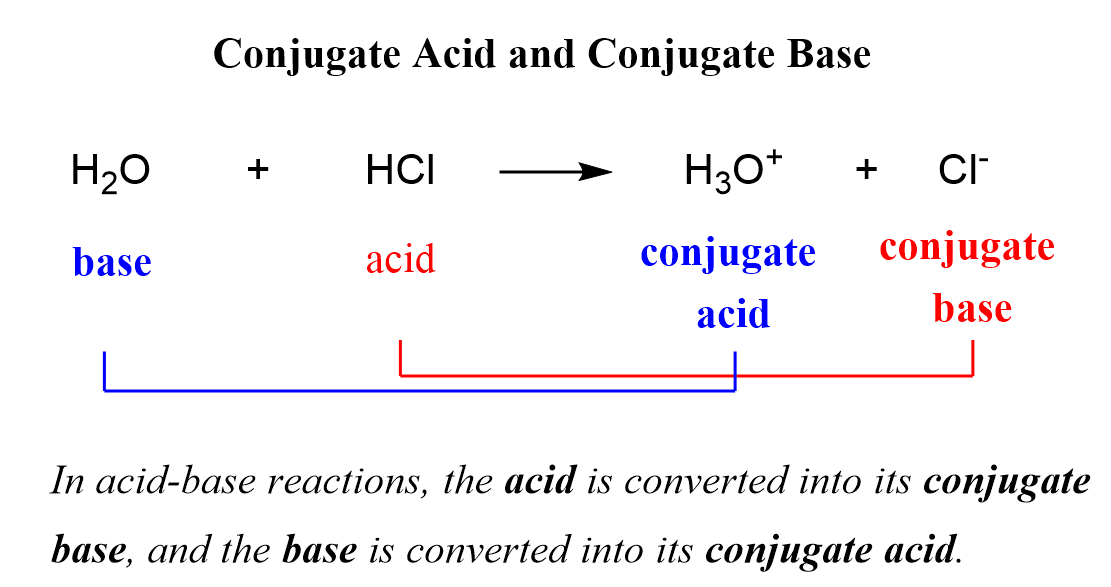
amphiprotic
a substance that can act as both a B-L acid and base (proton donor and acceptor)
sodium hydrogen carbonate
NaHCO3 + HCl ⇌ NaCl + H2O + CO2
potassium dihydrogen phosphate
KH₂PO₄
model the difference between strong/weak acids & concentrated/dilute acids
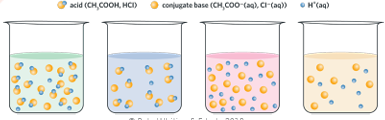
calculate the pH of the resultant solution when solutions of acids and/or bases are diluted or mixed
write acid/base reaction equation
find number of moles of either acid/base in excess
write the acid/base dissociation equation
use this to find concentration of H+/OH-
use this concentration to find pH/pOH
titration
Volumetric technique where the concentration of an analyte is determined by neutralisation with a standardised chemical.
titration curves — strong acid/strong base
As the base is added, OH- ions add to H+ ions to make water and reduce conductivity. As the equivalence point is passed the OH- ions start to build up again and increase solubility. The graph is the same for the reverse reaction.
conductivity graphs

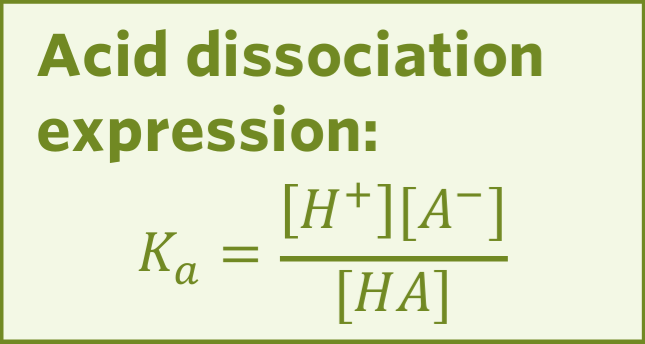
Ka
Ka is a measure of ionisation of an acid.
The larger the Ka the more the acid dissociates and therefore the stronger the acid.
Ka is completely acceptable in expressing the strength for strong acids, however it becomes unmanageable for weak and very weak acids.
This is where pKa becomes useful.
acid/base analysis techniques that are applied in industries
Many industrially produced fertilisers are the products of neutralisation reactions.
These reaction vessels can be monitored using industrial pH probes as a method of knowing when reactions are complete or which reagent needs increasing.
Ammonium nitrate is reacted with nitric acid in a neutraliser. Here the pH is measured to ensure that indeed the correct mixture has been achieved.
Ammonium nitrate has a pH of 5.3 as HNO3 is a strong acid.
Other aspects of this production need to be monitored as NH4NO3 is an extremely effective explosive that will combust without the presence of oxygen (it can use the oxygen present in the nitric oxides).
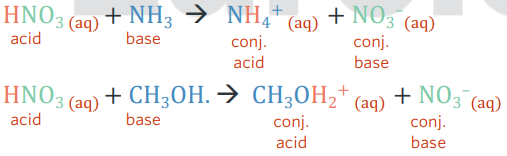
acid/base analysis techniques that are applied by Aboriginal and Torres Strait Islander Peoples
bull ants and bracken fern
Bracken fern (Pteridium esculentum) has alkaline juices in the leaves when crushed.
Bull ants inject painful formic acid as it stings.
The alkali juices of the bracken fern have been known to neutralise the formic acid.
HCOOH(aq) + BOH(aq) → BHCOO(aq) + H2O(l)
acid/base analysis techniques that are applied using digital probes and instruments
probes and digital measurements allow monitoring to be accurate, reliable and precise
require regular calibration
does not alter the analytes chemically
allows industrial processes to be automated or monitored on very large scales

aspirin back titration
Aspirin (CH3COOC6H4COOH) is a very weak acid that cannot be titrated traditionally
However it reacts with excess NaOH (calculate n(NaOH)initial) to produce NaCH3COO(aq) and HOC6H4COONa(aq) and H2O
The remaining NaOH can be titrated with standardised HCl(aq)
We can than calculate how much NaOH was used in the first reaction
Then the moles of aspirin can be calculated.
Then the mass of CH3COOC6H4COOH present in the tablet can be calculated
buffer
Buffer systems are chemical systems that resist changes in [H+] and [OH- ] – change is much less than expected.
Buffer systems consist of a weak acid and its conjugate base or a weak base and its conjugate acid.
HA(aq) ↔ H+ (aq) + A- (aq)
Both the acid and its conjugate base (or vice versa) are in solution in significant amounts.
The acid/base and its salt must be of higher concentrations than the concentration of the added acid/base. The higher the concentration the better the buffering action.
conduct a practical investigation to prepare a buffer and demonstrate its properties
a buffer can be prepared by mixing solutions of:
A weak acid and its salt.
A strong acid and the salt of a weak base.
A weak acid and a strong base.
buffers can be made on purpose or as the result of a titration.
strong acid/strong base reaction
strong acid + strong base → salt + water + energy
HA(aq) + BOH(aq) → AB(aq) + H2O(l) + energy
exothermic, as proton transfer is exothermic
strong acid/weak base reaction
strong acid + weak base → acidic salt
H+(aq) + B(aq) → HB(aq)
a conjugate acid of the weak base is formed — this is a neutralisation as excess H+ are used up but it leaves an acidic solution and does not become neutral — pH < 7
weak acid/strong base reaction
weak acid + strong base → water + conjugate base
HA(aq) + BOH(aq) → H2O(l) + AB(aq)
all H+ ions are used up with OH- ions and the conjugate base of the weak acid is formed
this is a neautralisation reaction as excess OH- ions are used up but it leaves a basic solution and does not become neutral — pH > 7
the conjugate base anion exists in equilibrium with the water to create OH- ions
properties of acids and bases
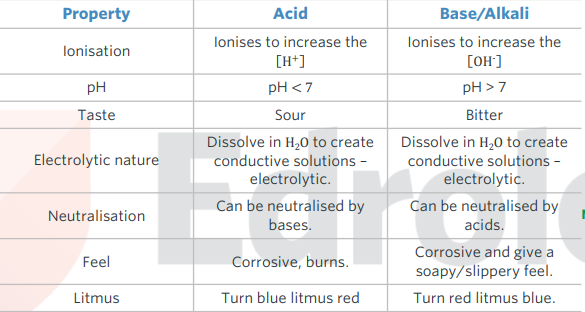
common indicators

volumetric analysis
Any practical analytical technique where the concentrations/volumes /molar quantities are calculated by reacting to known analytes and calculating the known volumes.
primary standard
A standard solution that has a known concentration and is prepared by the chemist.
secondary standard
A solution that has been prepared in the laboratory and has been titrated/ standardised against a primary standard solution.
pKa
pKa = -log(Ka)
back titration
Where a titrand which is unsuitable for titration can be reacted with excess acid or base and the resulting mixture is titrated to see how much acid or base is left over, thus how much was used up
titrand
the solution whose concentration is being determined
titrant
a solution of known concentration that is added to a solution of unknown concentration (the titrand) during a titration, to determine the concentration of the titrand
also known as the analyte
buffer capacity
the amount of acid/base that can be absorbed and still maintain the pH
Buffers are able to absorb the most pH/[H+]/[OH- ] change when:
pKa = pH and
[Acid] = [Conjugate base]
natural buffer systems
blood is a buffered solution at pH 7.35 - 7.45
buffered by H2CO3/NaHCO3 – if pH moves outside of this range, enzymes work less efficiently and metabolism will stop and ultimately end in death
the buffering happens in the equation:
H2CO3(aq) ↔ HCO3 - (aq) + H+ (aq)
By having H2CO3 present in solution with HCO3 - (present as NaHCO3):
As an acid enters the system, the H+ ions are absorbed by the reserve of HCO3 - ions and pH is maintained.
As bases enter the system the H2CO3 ion dissociates, donates a H+ ion to the base (making a salt or water) and pH is maintained.
As CO2 enters the blood as a result of respiration, it moves the two equilibria to the right, the bicarbonate reserves soak up the extra H+ ions.
Excess H2CO3(aq) can reverse the reaction back to CO2(g) and H2O(l) to be exhaled and excess HCO3 - (aq) can be removed via the kidneys to keep [acid] ≈ [base].