Identification of anions
1/6
Earn XP
Description and Tags
part 1 of 12.5 Identification of ions and gases
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
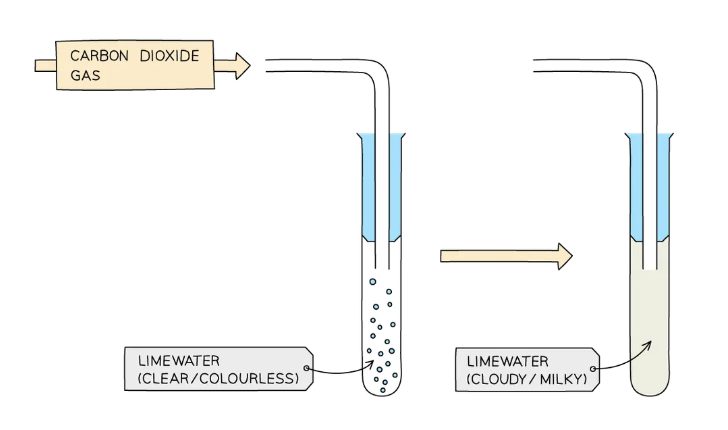
Test for carbonate ions CO32-
react sample with dilute acid
bubble gas released through limewater
if limewater turns cloudy, carbonate ion is present
Test for halide ions: Cl-
acidify sample with dilute nitric acid
add aqueous silver nitrate
a silver halide precipitate forms if a halide ion is present
the chloride ion forms a white precipitate of silver chloride
Test for halide ions: Br-
acidify sample with dilute nitric acid
add aqueous silver nitrate
a silver halide precipitate forms if a halide ion is present
the bromide ion forms a cream precipitate of silver chloride
Test for halide ions: I-
acidify sample with dilute nitric acid
add aqueous silver nitrate
a silver halide precipitate forms if a halide ion is present
the iodide ion forms a yellow precipitate of silver chloride
Test for nitrate ions: NO3-
reduce sample with aqueous NaOH and aluminium foil & heat gently
test for ammonia gas released
ammonia turns damp red litmus paper blue if present
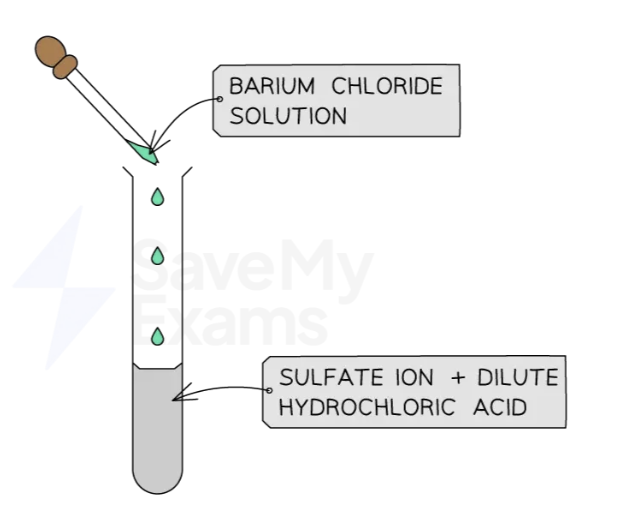
Test for sulfate ions: SO42-
Acidify sample with dilute nitric acid
Add a few drops of barium chloride solution
A white precipitate of barium sulfate is formed, if the sulfate ion is present
Test for sulfite ions: SO32-
Add dilute acid to sample
Warm the mixture gently
Bubble the gas released through potassium manganate(VII) solution
The potassium manganate(VII) solution changes from purple to colourless if the sulfite ion is present