D2.3 Water Potential
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
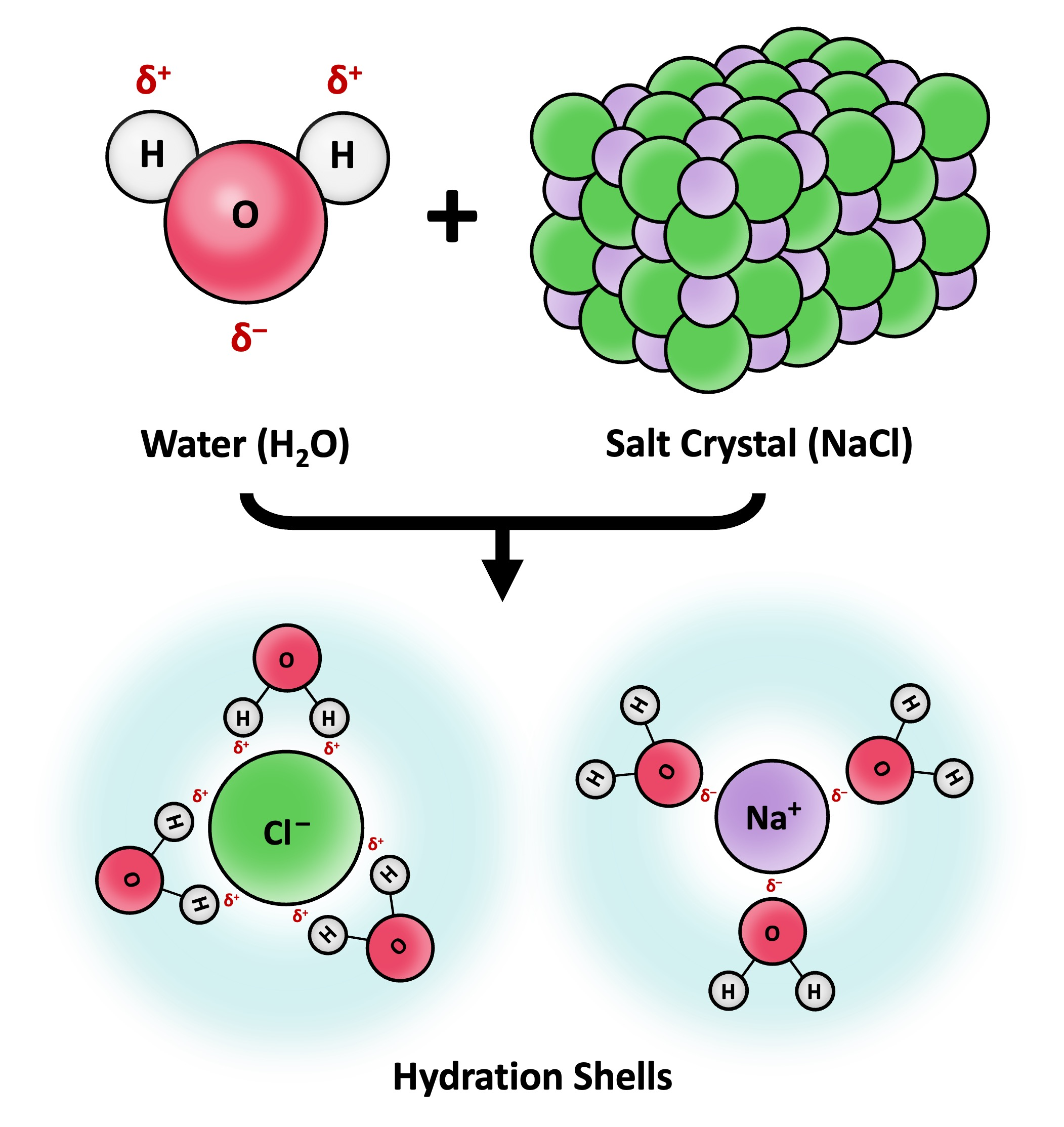
Define solvation
The process where a solvent surrounds and interacts with solute molecules or ions
Describe how water dissolves polar/charged substances (6)
Water’s ability to dissolve solutes & ions depend on its polarity:
Positive cations / solute parts attract water’s oxygen
Negative anions / solute parts attract water’s hydrogen
→ this forms hydrogen bonds or electrostatic attractions between water & polar molecules
After attraction, water molecules form hydration shells around solutes to prevent them from precipitating
This keeps ions dissolved & free to move → key for cellular processes
Define osmosis
The net movement of water molecules across a semi-permeable membrane, from a region of lower to higher solute concentration
Define isotonic
Two solutions with equal solute concentrations
Define hypotonic
A solution with a lower solute concentration
Define hypertonic
A solution with a higher solute concentration
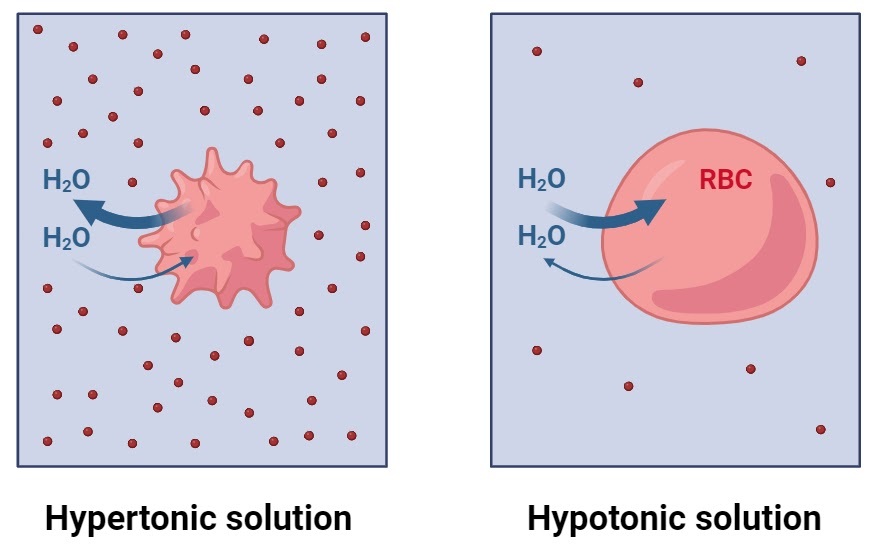
Describe osmosis between hypotonic & hypertonic solutions (2)
Individual water molecules move both ways across a membrane
But net movement is from hypotonic (less concentrated/more water) solution to hypertonic (more concentrated/less water) one → balance concentrations
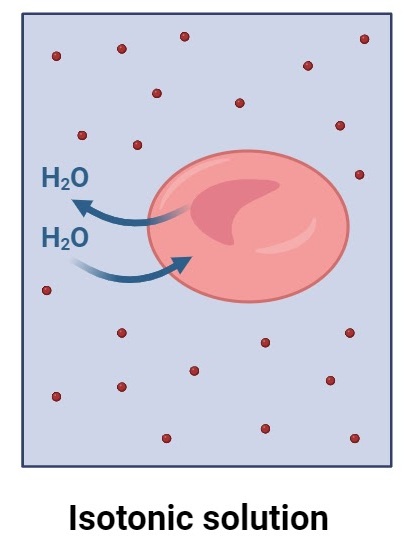
Describe osmosis between isotonic solutions
Water moves equally both ways → no net movement (dynamic equilibrium)
Describe how osmosis works (3)
How osmosis works:
Cells have a plasma membrane separating their cytoplasm from outside fluids
The membrane is highly permeable to water, but less permeable to solutes
When solute concentrations differ inside & outside the cell, water moves across the membrane to less solute-concentrated region
Describe directions of water movement in different solutions
Solution | Direction of water movement |
Hypotonic (lower solute) | Moves into the cell |
Hypertonic (higher solute) | Moves out of the cell |
Isotonic (equal solute) | Moves both ways equally (dynamic equilibrium) |
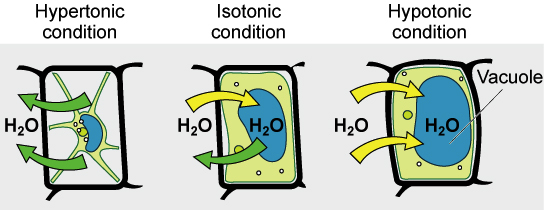
Compare the effect of hypotonic & hypertonic solutions on cells w/o cell wall
Solution | Effect |
Hypotonic |
|
Hypertonic | As water moves out of cell, it shrinks & shrivels → reducing mass & length |
How are some organisms adapted for osmosis? (3)
1) Freshwater unicellular organisms -
Lives in hypotonic environments (water drawn in by osmosis)
Although they lack cell walls, they use contractile vacuoles to pump out excess water → prevent bursting
2) Multicellular organisms -
Must maintain isotonic tissue fluid (has same solute concentration as cells) → prevents swelling/shrinkage of cells from affecting organ function
Compare the effect of hypotonic & hypertonic solutions on cells w/ walls
Effects in hypotonic vs hypertonic solutions:
Solution | Effect (plant cells) |
Hypotonic |
|
Hypertonic |
|
Describe medical applications of isotonic solutions (4)
Application | Purpose |
Intravenous (IV) drip | Rehydrates patients, restores fluid balance in blood → most common is normal saline (0.9% sodium chloride) |
Organ preservation before transplantation | Frozen saline slush cools hearts, kidneys, & other organs |
Wound and skin irrigation | Cleans wounds to prevent infection |
Moistening damaged skin before grafts | Keeps tissue healthy and prevents drying out |
Eye drops | Provides safe moisture to eyes without irritation |
Define water potential
The potential energy of water relative to pure water per unit volume
What symbol represents water potential?
Psi (Ψ)
What units are water potential measured in?
Kilopascals (kPa) or megapascals (MPa)
Explain why water potential is relative to pure water (2)
Absolute water potential is immeasurable, so values are relative to pure water
Pure water is at atmospheric pressure & 20°C → water potential remains at 0kPa
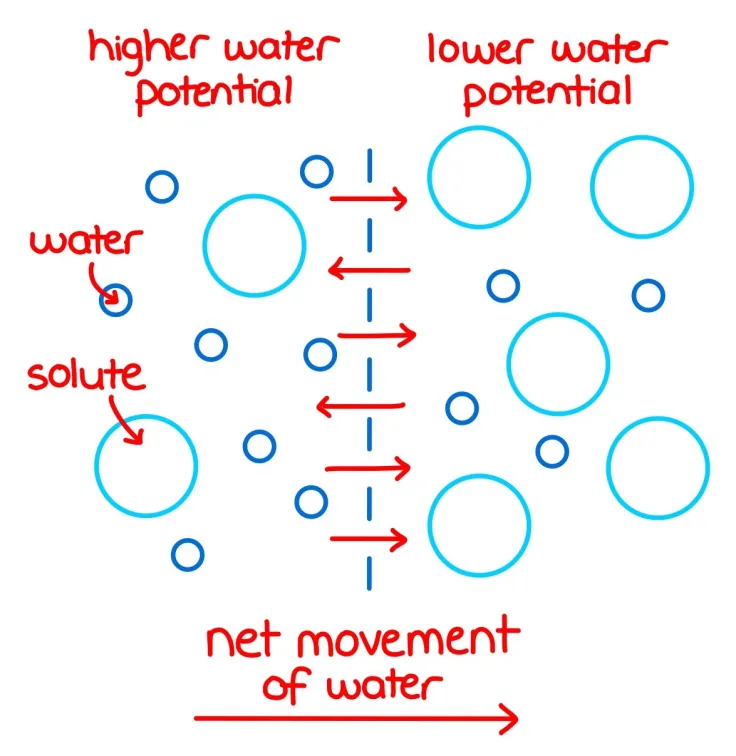
Why does water move from high to low water potential? (3)
1) Energy minimisation - Minimises potential energy, making the system more stable
2) Balancing solute concentrations - Dilutes solutes to equalise solute concentrations across membranes
3) Pressure differences - Positive pressure drive water outward, while negative pressure pulls water inward
Give the equation for water potential (include symbols)
Water potential (Ψw) = Solute potential (Ψs) + Pressure potential (Ψp)
How does solute potential affect water potential?
Adding solutes makes Ψs more negative
Solutes reduce free water molecules, decreasing water potential
Pure water has Ψs = 0 (no solutes)
How does pressure potential affect water potential? (give examples)
Positive pressure (above atmospheric) → increases water potential (Ψp > 0)
Example: Turgor pressure in healthy plant cells
Negative pressure (below atmospheric) → decreases water potential (Ψp < 0)
Example: Tension in xylem vessels pulling water upward
For pure water/at atmospheric pressure, Ψp = 0
Define solute (osmotic) pressure
The effect solutes in a solution have on water potential
Define pressure potential
The physical pressure exerted onto water
What happens to plant tissues in hypotonic solutions? (6)
Hypotonic solutions have higher water potential than plant cells
This causes water to move into cells by osmosis
Cytoplasm’s high solute concentration lowers solution’s solute potential → decreases water potential
Water intake increases pressure potential & cytoplasm volume → turgor pressure
Cells become turgid (swollen & firm)
When internal water potential = external, water movement stops
What happens to plant tissues in hypertonic solutions? (6)
Hypertonic solutions have lower water potential than plant cells
Initially, cells have positive pressure potential due to turgor
But as water leaves, pressure potential decreases
This causes cells to become flaccid (limp)
Continued water loss causes plasmolysis: plasma membrane pulls away from cell wall
At extreme dehydration, internal water potential = external, stopping water movement