BIOC 303: Lipid Metabolism
1/62
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
63 Terms
Fatty Acid Transport into Mitochondria: Background
the enzymes of fatty acid oxidation in animal cells are located in the mitochondria matrix
short and medium chain fatty acids (12 or less C’s) enter the mitochondria without the help of membrane transporter
long chain fatty fatty acids (14 or more C’s) cannot pass directly thru the mitochondrial membranes, they must be transported thru the carnitine shuttle
majority of FFAs obtained in the diet or released from adipose tissue
Fatty Acyl-CoA Synthetase
catalyzes the formation of a thioester linkage between the fatty acid carboxyl group and the thiol group of coenzyme A to yield a fatty acyl-CoA
activates the fatty acid
costs 2 ATP
coupled to the cleavage of ATP to AMP and PPi
an isozyme specific for long-chain fatty acids and present in the outer mitochondrial membrane
Fatty Acyl-CoAs
high energy compounds
their hydrolysis to FFAs and CoA has a large negative standard free-energy change
formation of fatty acyl-CoA is made more favorable by the hydrolysis of 2 high-energy bonds in ATP; the pyrophosphate is immediately hydrolyzed by inorganic pyrphosphatase
fatty acyl-CoA ester formed on the cystolic side of the OMM can be transported into the mitochondrion and oxidized to produce ATP, or they can be used in the cytosol to synthesize membrane lipids
those destined for mitochondrial oxidation must be attached to carnitine to be shuttled across the IMM
The Oxidation of Long Chain FAs to Acetyl-CoA
serves as a central energy-yielding pathway in many organisms and tissues
provides as much as 80% of the energetic needs in mammalian heart and liver
provides >40% of the daily energy requirement
electrons removed from fatty acids during oxidation pass thru the respiratory chain, driving ATP synthesis
acetyl-coA produced from the fatty acids may be completely oxidized to CO2 in the citric acid cycle
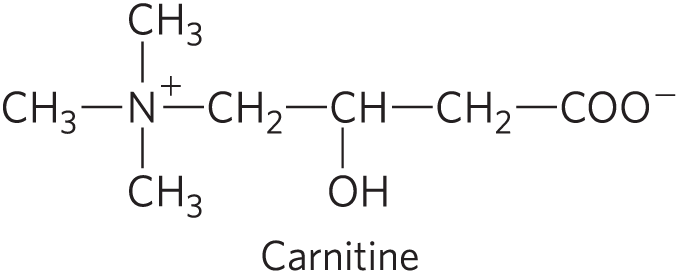
Carnitine
Compound that transports fatty acyl-CoAs destined for mitochondrial oxidation across the inner mitochondrial mebrane
small
modified amino acid
charged
cells can easily recognize this
Carnitine Acyltransferase 1 (CAT1)
catalyzes a transesterification reaction to transiently attach a fatty acyl-CoA to the hydroxyl group of carnitine to form fatty acyl-carnitine
inhibitied by malonyl-coA, the first intermediate in fatty acid synthesis
prevents the simultaneous synthesis and degradation of fatty acids
exchanges the S-CoA on the fatty acyl-CoA for carnitine in the OMM
S-CoA is a good leaving group
Acyl-Carnitine/Carnitine Cotransporter
allows the passive transport of the fatty acyl-carnitine ester
located in the IMM
moves one molecule of carnitine from the matrix to the intermembrane space as one molecule of fatty acyl-carnitine moves into the matrix
Carnitine Acyltransferase 2 (CAT2)
transfers the fatty acyl group from carnitine back to coenzyme A to regenerate fatty acyl-coA and free carnitine
located on inner face of IMM
the carnitine is now available to be transferred back thru the acyl carnitine/carnitine cotransporter to be used to shuttle the next fatty acid acros
Two Pools of Coenzyme A
one pool in the cytosol, on in the mitochondria
coenzyme A in the mitochondrial matrix is largely used in oxidative degradation of pyruvate, fatty acids and some amino acids
cytosolic coenzyme A is used in the biosynthesis of fatty acids
Two Pools of Fatty Acyl-CoA
one pool in the cytosol, on in the mitochondria
fatty acyl-coA in the cytosolic pool can be used for membrane lipid synthesis of can be moved into the mitochondrial matrix for oxidation and ATP production
conversion to the carnitine ester commits the fatty acyl moiety to the oxidative fate
mitochondrial fatty acyl-coA undergoes β-oxidation
Carnitine Shuttle is a Major Control Point
carnitine-mediated entry is the rate-limiting step for oxidation of fatty acids in mitochondria
β-Oxidation: Details
fatty acids undergo oxidative removal of successive two-carbon units in the form of acetyl-coA, starting from the carboxyl end of the fatty acyl chain
# of passes: n Carbons / 2 -1
-1 because the last two carbons will remain as acetyl-CoA
works for saturated (even C’s) and unsaturated fatty acids
# of passes for odd numbered fatty acids: ( n Carbons - 3) / 2
formation of each acetyl-coA requires removal of 4 hydrogen atoms (two pairs of electrons and four H+) from the fatty acyl moiety by dehydrogenases
each acetyl-CoA can turn the TCA cycle once, producing 10 ATP equivalents
in total 14 ATP generated with β oxidation
β-Oxidation: Step 1
oxidation (dehydrogenation) reaction of fatty acyl-CoA forms a double bond between the ⍺ and β carbons (C2 and C3)
yields trans-∆2-enoyl-coA
catalyzed by acyl-coA dehydrogenase
the new double bond has the trans configuration, whereas the double bonds in naturally occurring unsaturated fatty acids are normally in the cis configuration
analagous to succinate dehydrogenation in the citric acid cycle
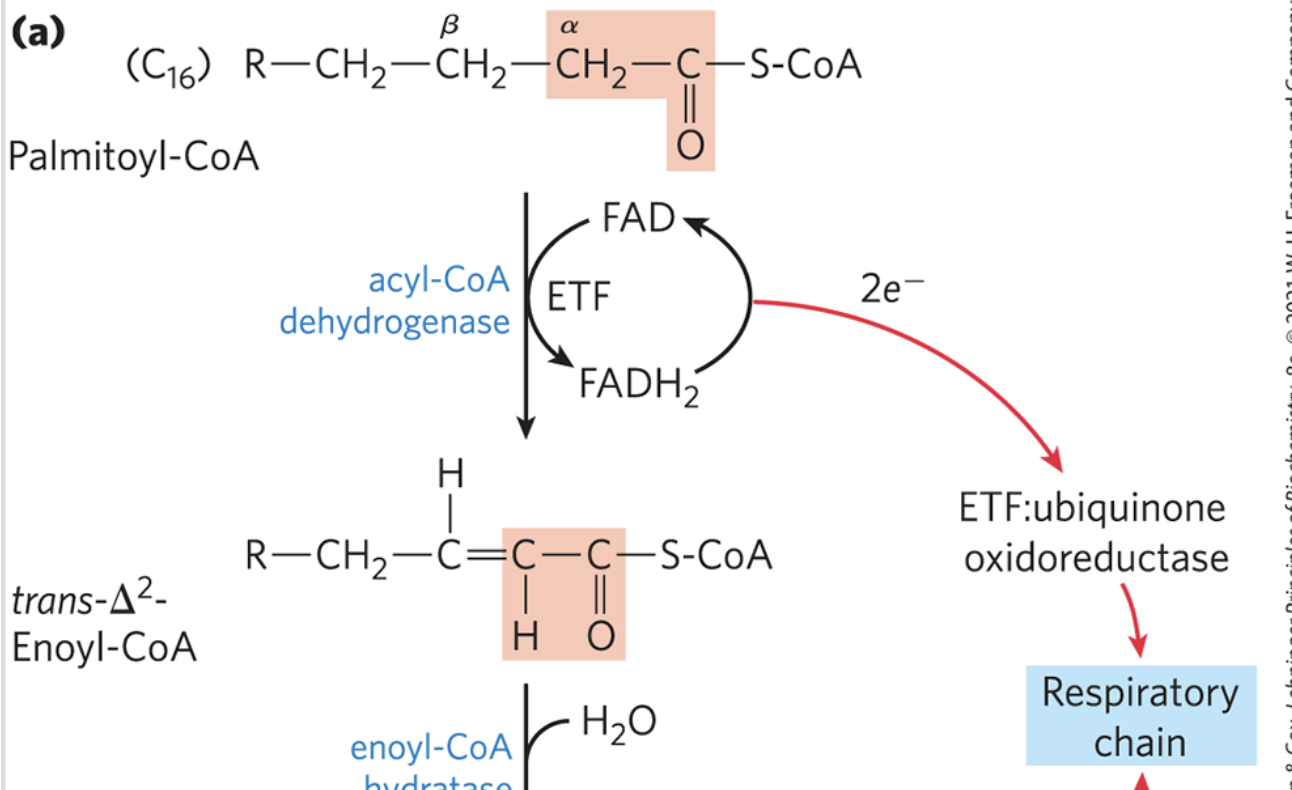
β-Oxidation: Acyl-CoA Dehydrogenase
flavoprotein with tightly bound FAD
FAD gets reduced in this reaction, and FADH2 can pass these two electrons to the ETC to generate energy in the form of 1.5 ATP
3 types of this isozyme, specific for a range of fatty acyl chain lengths:
very long chain acyl dehydrogenase (VLCAD): 12-18 carbons
medium chain (MCAD): 4-14 carbons
short chain (SCAD): 4-8 carbons
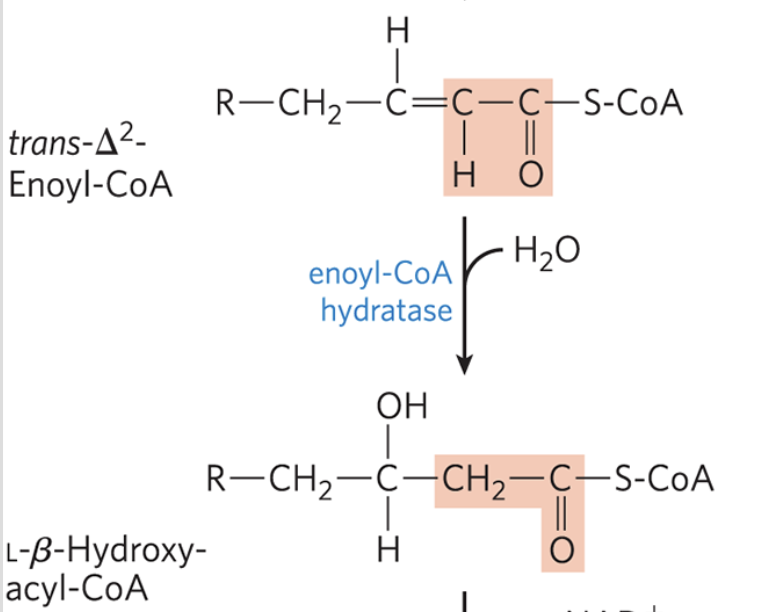
β-Oxidation: Step 2
water is added to the double bond of the trans-∆2-enoyl-CoA to form the L-stereoisomer of β-hydroxyacyl-CoA (3-hydroxyacyl-CoA)
catalyzed by enoyl-CoA hydratase and it only works on trans double bonds
analogous to fumarase reaction in the citric acid cycle
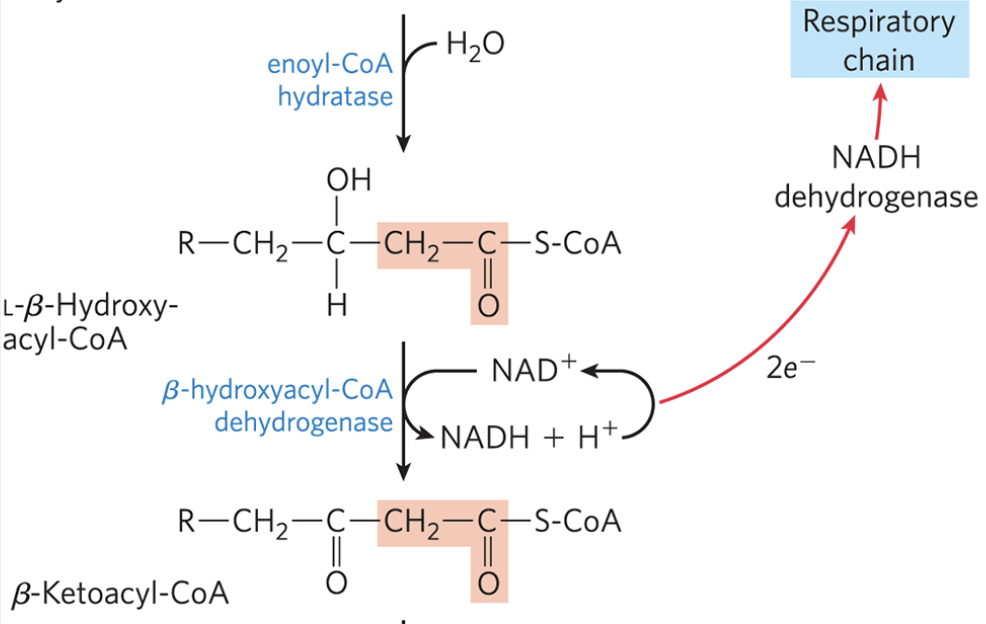
β-Oxidation: Step 3
L-β-hydroxyacyl-CoA is dehydrogenated to form β-ketoacyl-CoA
catalyzed by β-hydroxylacyl-CoA dehydrogenase and specific for the L-isomer
NAD+ is the electron acceptor, and the NADH formed in the reaction donates its electrons to the ETC, leading to the formation of 2.5 ATP
analogous to the malate dehydrogenase reaction in TCA cycle
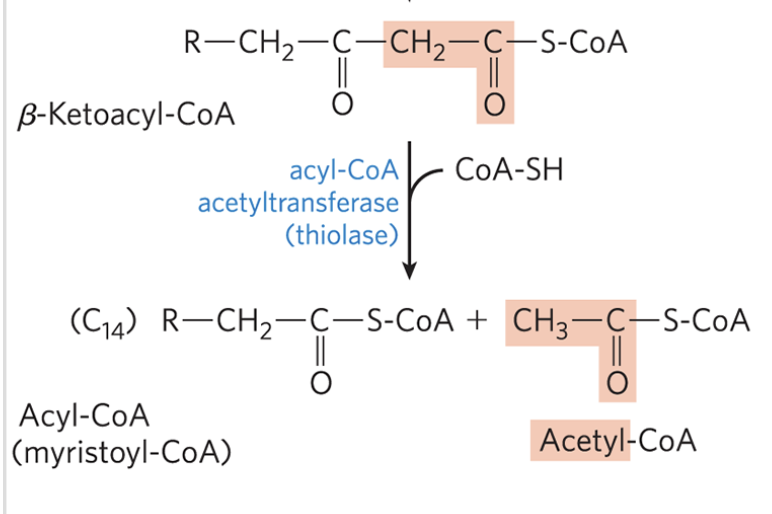
β-Oxidation: Step 4
catalyzed by acyl-CoA acetyltransferase (thiolase)
reaction of β-ketoacyl-CoA with a free coenzyme A to split off the carboxyl-terminal two-carbon fragment of the original fatty acid as acetyl-CoA
the other product is the coenzyme A thioester of the fatty acid, now shortened by two carbon atoms
reverse claisen condensation reaction
Trifunctional Protein (TFP)
a multienzyme complex associated with the inner mitochondrial membrane that catalyzes steps 2-4 of β-oxidation for fatty acyl chains of 12+ carbons (long chains)
heterooctamer of ⍺4β4 subunits
each ⍺ subunit contains 2 activities, the enoyl-coA hydratas and β-hydroxylacyl-CoA dehydrogenase
β subunits contain the thiolase activity
the tight association of these enzymes alllows efficient substrate channeling from one active site to the next, without diffusion of the intermediates away from the enzyme surface
when TFP has shortened the fatty acyl chain to 12 or fewer carbons, further oxidations are catalyzed by medium/short chain enzymes
The Chemical Logic of the β-Oxidation Sequence
the first 3 reactions of β-oxidation create a much less stable C-C bond
the ⍺ carbon (C2) is bonded to two carbonyl carbons (the β-ketoacyl-CoA intermediate)
the ketone function on the β carbon (C3) makes it a good target for nucleophilic attack by the –SH of coenzyme A, catalyzed by thiolase
the acidity of the ⍺-hydrogen and the resonance stabilization of the carbanion generated by the departure of this hydrogen makes the terminal –CH2–CO–S-CoA a good leaving group
facilitating breakage of the ⍺-β bond
One Pass of β-Oxidation
Fatty acyl-CoA + CoA + FAD + NAD+ + H2O →
shortened fatty acyl-CoA + acetyl-CoA + FADH2 + NADH + H+
1 acetyl-CoA, 2 pairs of electrons, 4 protons are removed from the long-chain fatty acyl-CoA
4 molecules of ATP are formed for each 2-carbon unit removed in one pass thru the sequence
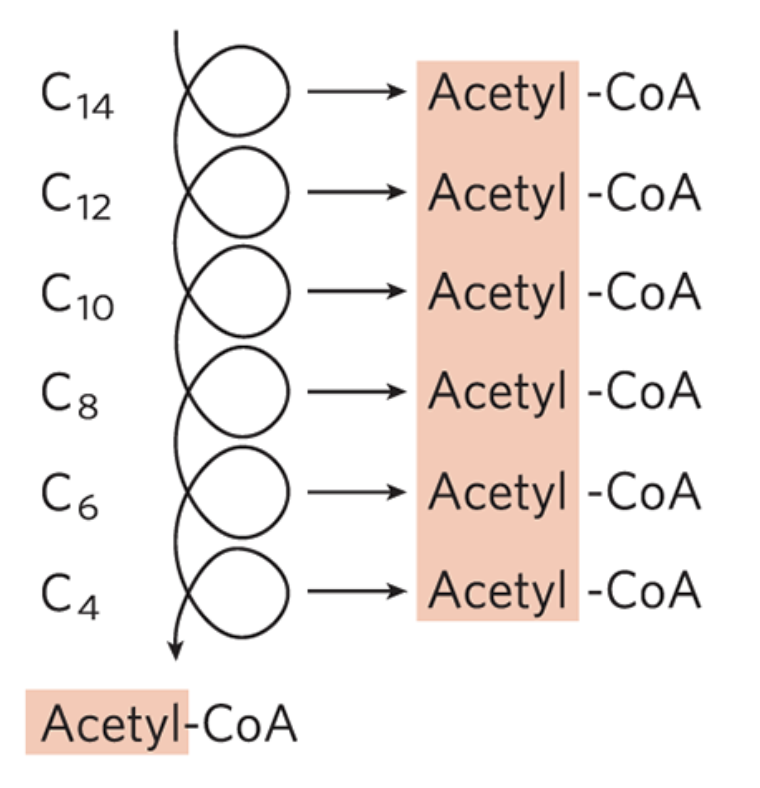
Repetition of β-Oxidation
the shortened fatty acyl-CoA reenters the β-oxidation sequence for removal of another, and then another, acetyl-CoA
Genetic Defects in Fatty Acyl-CoA dehydrogenases
inability to oxidize fatty acids from TAGs has serious health consequences
individuals with 2 mutant MCAD alleles cannot oxidize fatty acids of 6-12 carbons
lead to build up of medium chain fatty acids; long chains that have been shortened and transferred to MCAD will also not be able to do processes with MCAD
symptoms include fatty liver, high blood levels of octanoic acid (8:0), coma and death
Water in β-Oxidation
each pair of electrons transferred from NADH or FADH2 to O2 yields one H2O (“metabolic water”)
2 H2O produced per cycle
Further Oxidation of Acetyl-CoA
the acetyl-CoA produced from β-oxidation of fatty acids can be oxidized to CO2 and H2O by the TCA cycle
the second stage of fatty acid oxidation
n/2 Acetyl-coA + n O2 + 10n ADP + 10n Pi → n CO2 + 10n ATP + n H2O
n = number of carbons in the original fatty acyl-CoA chain
From β-oxidation to end of TCA for even, saturated chains
-2 ATP from this equation because activating a fatty acid takes 2 ATP
fatty acyl-CoA (Cn) + (3n/2-1) O2 + (7n - 4) ADP + (7n - 4) Pi → CoA + (7n - 4) ATP + n CO2 + (3n/2 -1) H2O
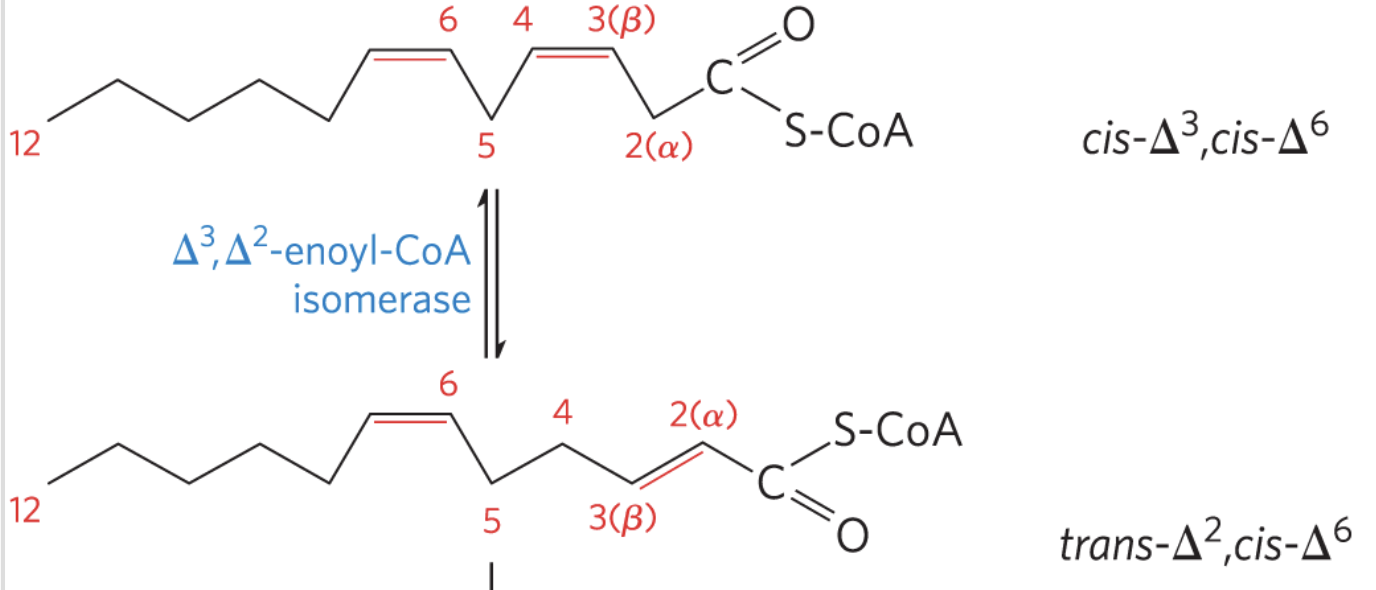
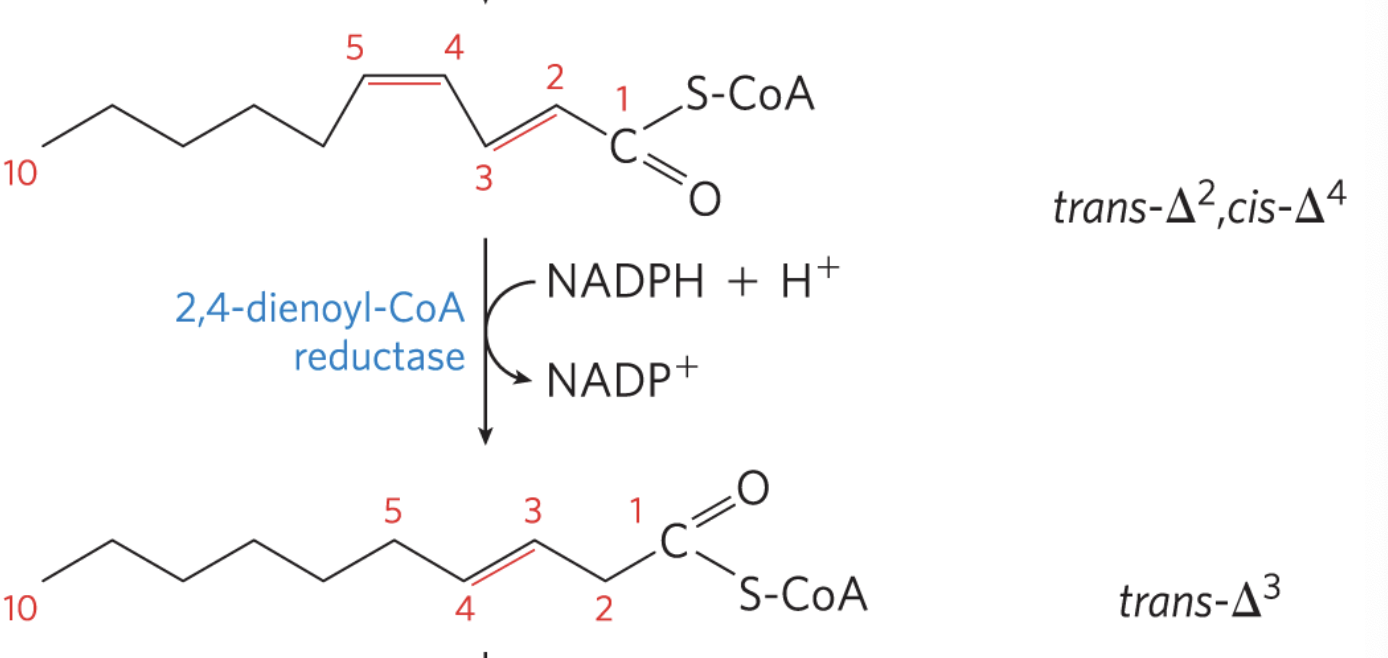
Oxidation of Unsaturated Fatty Acids
most naturally occurring fatty acids have cis double bonds, and cannot be acted upon by enoyl-CoA hydratase
requires two additional enzymes to transform into substrates for β-oxidation:
enoyl-CoA isomerase: converts cis double bonds to trans
only this is needed for monosaturated fats
2,4-dienoyl-CoA reductase: reduces cis double bonds
required for PUFAs
Enoyl-CoA Isomerase Figure
2-4-Dienoyl-CoA Reductase
Oxidation of Odd-Number Fatty Acids
oxidized in the same pathway as even-number fatty acids, beginning at the carboxyl chain
the substrate for the last pass thru the β-oxidation sequence is a fatty acyl-CoA with a five carbon fatty acid
when oxidized and cleaved, the products are acetyl-CoA and propionyl-CoA
Propionate
3 carbon compounds formed by cattle and other ruminant animals during carbohydrate fermentation
the propionate is absorbed into the blood and oxidized by the liver and other tissues
CH3–CH2–COO-
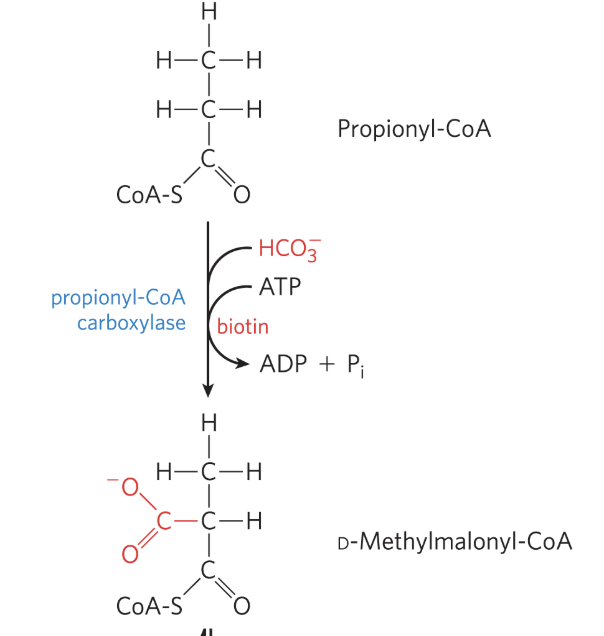
Oxidation of Propionyl-CoA: Step 1
catalyzed by proprionyl-CoA carboxylase, which contains cofactor biotin
propionyl-CoA is carboxylated to form D-methylmalonyl-CoA
bicarbonate ion (HCO3-) is activated by attachment to biotin before its transfer to the propionate moiety
formation of the carboxybiotine intermediate requires ATP
Absence of Functional Propionyl-CoA Carboxylase
leads to an accumulation of propionyl-CoA in mitochondria, depleting the available supply of coenzyme A for continuing β-oxidation
propionyl-CoA is esterified to carnitine, transported out the mitochondria via the carnitine shuttle and released to blood as propionate
this severely acidifies blood and urine
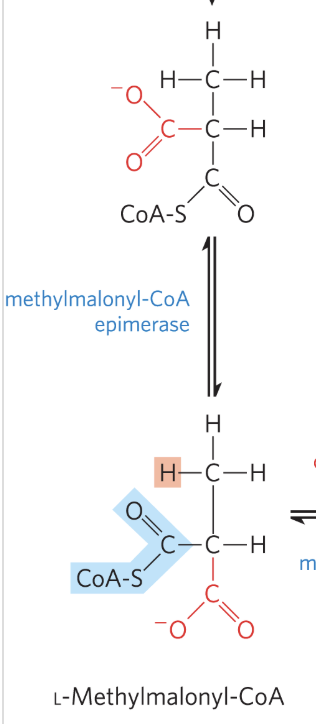
Oxidation of Propionyl-CoA: Step 2
catalyzed by methylmalonyl-CoA epimerase
D-methylmalonyl-CoA is epimerized to its L stereoisomer
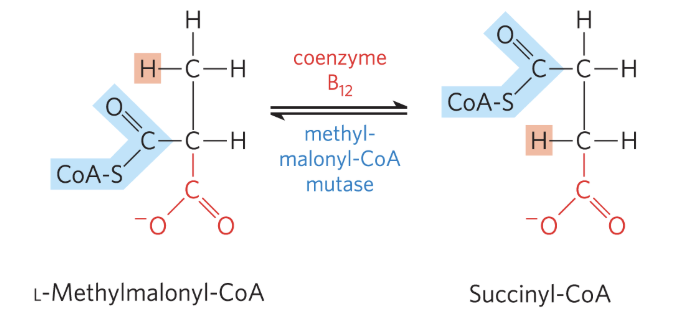
Oxidation of Propionyl-CoA: Step 3
catalyzed by methylmalonyl-CoA mutase
requires coenzyme B12
L-methylmalonyl-CoA undergoes intramolcular rearrangement to form succinyl-CoA, which can enter the TCA cycle
net gain of oxidation of priopionyl-CoA = 4 (1 GTP, 1 FADH2 = 1.5 ATP, 1 NADH = 2.5 ATP)
was 5, but -1 from step 1
Fatty Acid Oxidation is Tightly Regulated
oxidation of fatty acids consumes valuable energy stores, and is regulated to only occur when organism’s require energy
in the liver, fatty acyl-CoA formed in the cytosol has 2 major pathways to it:
β oxidation by enzymes in mitochondria
conversion into triacylglycerols and phospholipids by enzymes in the cytosol
the pathway taken depends on the rate of transfer of long-chain fatty acyl-CoA into mitochondria
the carnitine by which fatty acyl groups are carried into the mitochondrial matrix as fatty acyl carnitine is the rate limiting step for fatty acid oxidation
important point of regulation
once fatty acyl groups have entered the mitochondria, they are committed to oxidation to acetyl-CoA
Peroxisomes
organelles found in plants and animals
the major site of β oxidation in plant cells
β Oxidation in Peroxisomes
the intermediates for β oxidation of fatty acids are coenzyme A derivatives
process consists of 4 steps like in mitochondrial β oxidation: 1) dehydrogenation, 2) hydration, 3) oxidation of the β-hydroxyacyl-CoA to a ketone, 4) thiolytic cleavage by coenzyme A
high concentrations of fats in the diet result in increased synthesis of the enzymes of peroxisomal β oxidation in the liver
liver peroxisomes do not contain the enzymes of the citric acid cycle and cannot catalyze the oxidation of acetyl-CoA to CO2
long chain/branched fatty acids are catabolized in peroxisomes to shorten chain producst, which are exported to mitochondria and completely oxidized there
Differences between Peroxisomal and Mitochondrial Pathways
Chemistry of the first step
in peroxisomes, the flavoprotein acyl-CoA oxidase that introduces the double bond passes electron directly to O2, producing H2O2
this oxidant is immediately cleaved to H2O and O2 by catalase
in peroxisomes, the energy released in step 1 is not conserved as ATP, but dissipated as heat
in mitochondria, the electrons removed in step 1 pass thru the ETC to O2, producing water and ATP
Specificity of Chain Length
the peroxisomal system is more active on very-long chain, and branched fatty acids
Genetic Defects in Peroxisomal Oxidation
Zellweger syndrome: unable to make peroxisomes, and therefore lack all metabolism related to peroxisomes
X-linked adrenoleukodystrophy (XALD): peroxisomes fail to oxidize VLCFA, due to the lack of function of the ABCD1 transporter in the peroxisomal membrane
Both defects lead to accumulation in the blood of VLCFA, and is a diagnostic sign of these disorders
leads to neurological disorders including demyelination → motor impairment, memory loss and seizures
Treatments: bone marrow transplants, lentiviral therapies, Lorenzo’s oil
Malonyl-CoA Formation from Acetly-CoA: Details
irreversible 3 step process that occurs in the cytoplasm
catalyzed by acetyl-CoA carboxylase
contains a biotin prosthetic group covalently bound in amide linkage to the 𝞢-amino group of a Lys residue
Malonyl-CoA Formation from Acetly-CoA: Step 1
a carboxyl group from HCO3- is transferred to biotin in an ATP dependent reaction
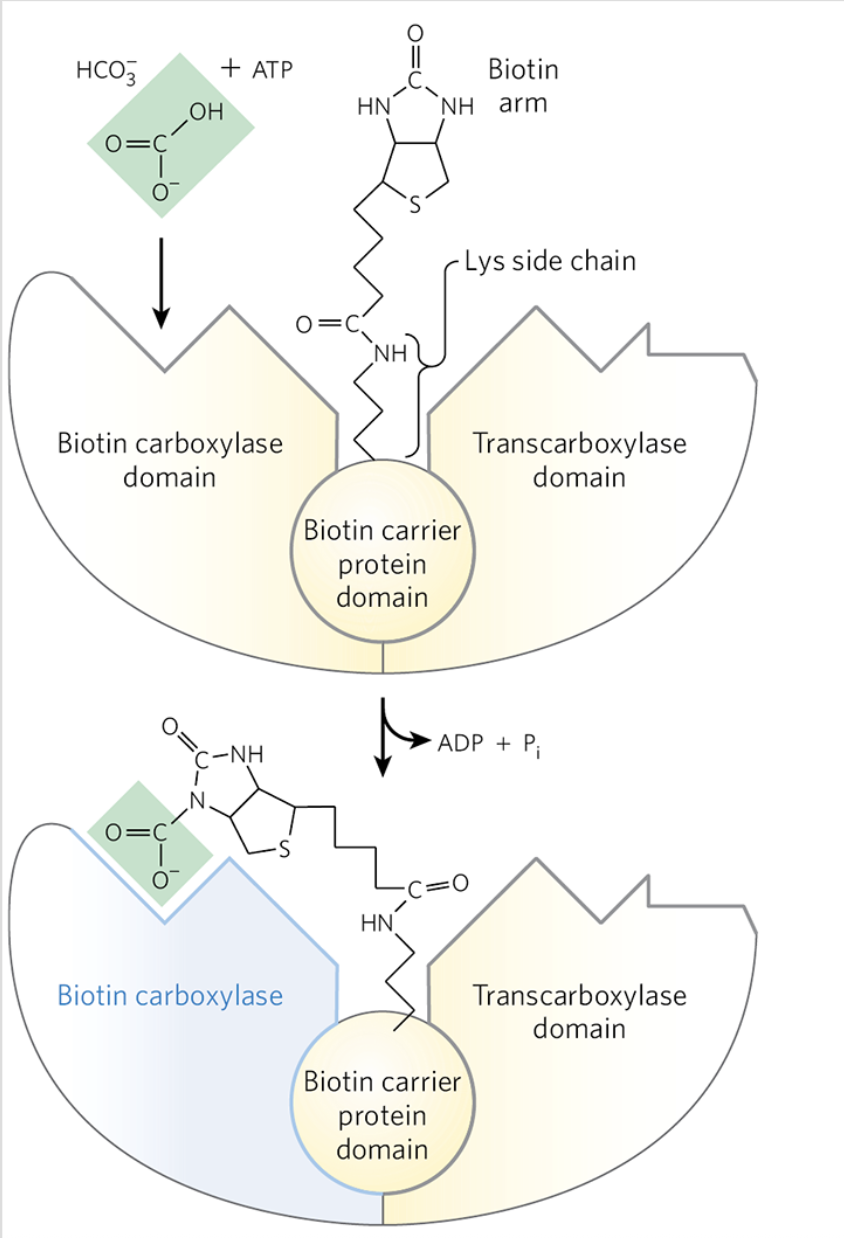
Malonyl-CoA Formation from Acetly-CoA: Step 2 + 3
the carboxyl group is carried by the biotin to a different active site, where the CO2 is transferred to acetyl-CoA in the third and final step to yield malonyl-CoA
this carboxylation step renders the next step (condensation in FAS) more favorable thermodynamically
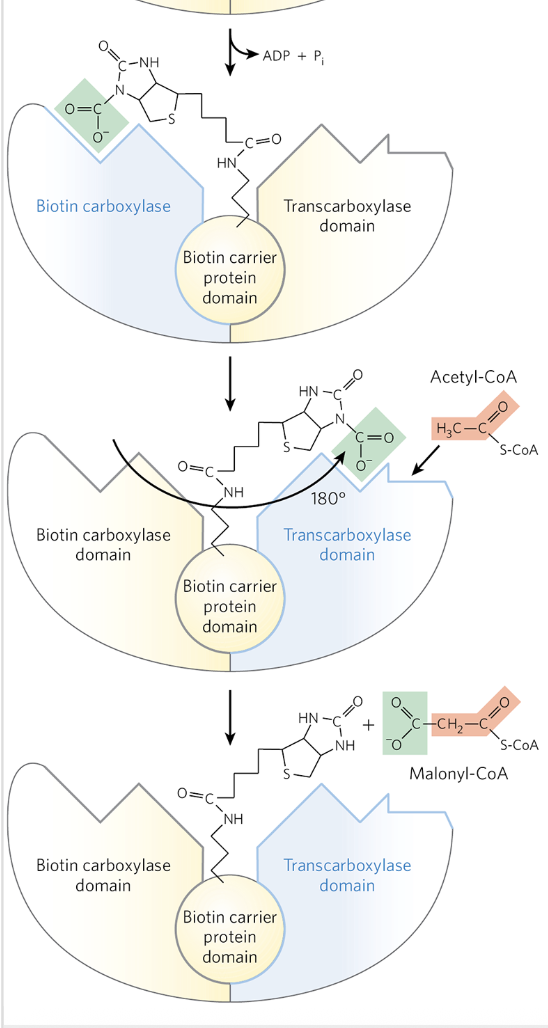
Why is bicarbonate used to activate Acetyl-CoA
good leaving group once attached
it’s around, it forms spontaneously when CO2 dissolves in water
biology is complex and doesn’t always use the most straightforward pathways
Fatty Acid Synthase (FAS1)
catalyzes assembly of long carbon chains of fatty acids in the cytosol thru a repeating 4-step sequence
begins with malonyl-CoA and acetyl-CoA
each passage thru the cycle elongates chain by 2 carbons
the product of each cycle feeds back into the cycle as the starting material for the next condensation step with malonyl-CoA
7 active sites to catalyze the 4 step cycle
FAS1 Reactions
a condensation reaction is followed by a reduction-dehydration-reducation sequence to convert the C3 carbonyl to a methylene
the last 3 steps are the chemical reverse of the OHO sequence in β oxidation of fatty acids
the electron-cofactor and activating groups differ from β oxidation
the reducing agent in FAS is NADPH and the activating groups are two difference enzyme-bound –SH groups
fatty acid synthesis leads to a single product
FAS Active Sties
the active site for each enzyme is found in a separate domain within the larger polypeptide (FAS1)
throughout FAS, the intermediates remain covalently attached as thioesters to one of two thiol groups
–SH group of a Cys residue in β-ketoacyl-ACP synthase
–SH group of ACP
hydrolysis of thioesters is highly exergonic and the energy released helps make steps 1 and 5 thermodynamically favorable
Acyl Carrier Protein (ACP)
as it goes thru the FAS cycle, the acyl group is covalently linked to ACP, which shuttles it from one active site to another in sequence
part of FAS1 polypeptide
no intermediates are released
when the chain length reaches 16 carbons, the product, palmitate (16:0) leaves the cycle (7 cycles)
C16 and C15 of palmitate are derived from acetyl-CoA, the rest of the carbon atoms in the chain are derived from acetyl-CoA via malonyl-CoA
4’-phosphopantetheine
ACP is the shuttle that holds the system together, containing the prosthetic group 4’-phosphopantetheine, also found in coenzyme A
serves as a flexible arm, tethering the growing fatty acyl chain to the surface of the fatty acid synthase complex while carrying the reaction intermediates from one enzyme active site to the next
Fatty Acid Synthase Receives the Acetyl and Malonyl Groups (before step 1)
before the condesation rxns of FAS can begin, the two thiol groups on the enzyme complex must be charged with the correct acyl groups
the acetyl group of acetyl-CoA is transferred to ACP in a rxn catalyzed by the malonyl/acetyl-CoA ACP transferase (MAT) domain of the multifunctional polypeptide
the acetyl group is then transferred to the Cys–SH group of the β-ketoacyl-ACP synthase (KS)
transfer of the malonyl group from malonyl-CoA to the –SH group of ACP
also catalyzed by MAT
FAS: Step 1 Condensation
Claisen condesation of activated acetyl and malonyl groups to form acetylacetyl-ACP, molecule of CO2 simultaneously produced
catalyzed by β-ketoacyl-ACP synthase
the acetyl group is transferred from the Cys-SH group of the enzyme to the malonyl group on the –SH of ACP, becoming the methyl terminal of the new acetoacetyl group
the C atom of CO2 produced is the same one that was introduced into malonyl-CoA from HCO3- in the acetyl-CoA carboxylase reaction
therefore, CO2 is only transiently in covalent linkage during FAS
its removed as each two-carbon unit is added
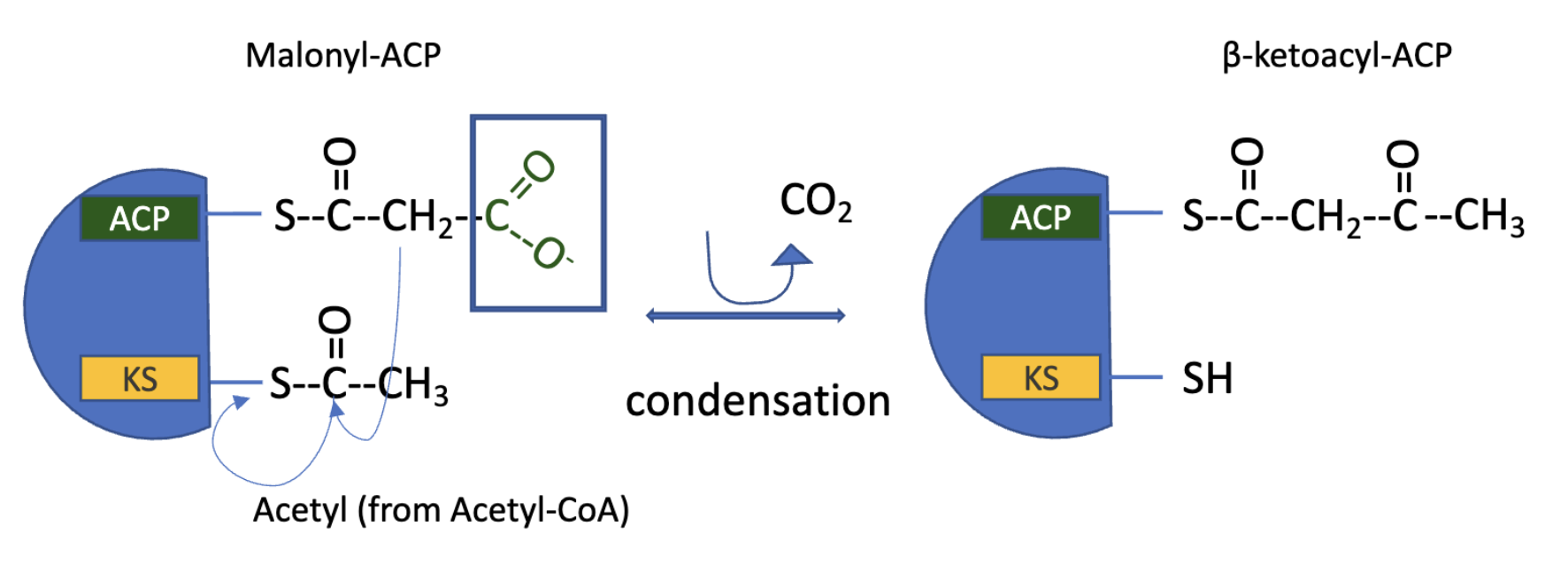
Why add CO2 to make malonyl group only to lose it during FAS?
the use of activated malonyl groups rather than acetyl group makes the condensation reactions thermodynamically favorable
the methylene carbon of malonyl group is sandwiched between carbonyl and carboxyl carbons, which forms a good nucleophile
decarboxylation of the malonyl group facilitates nucleophilic attack of the methylene carbon on the thioester linking the acetyl group to β-ketoacyl-synthase, displacing the enzyme’s SH group
FAS: Step 2 Reduction
reduction of the carbonyl group
acetoacetyl-ACP undergoes reduction of the carbonyl group at C3 to form D-β-hydroxybutyryl-ACP
note different stereoisomer than the intermediate in fatty acid oxidation (L-β-hydroxyacyl)
catalyzed by β-ketoacyl ACP reductase
electron donor: NADPH
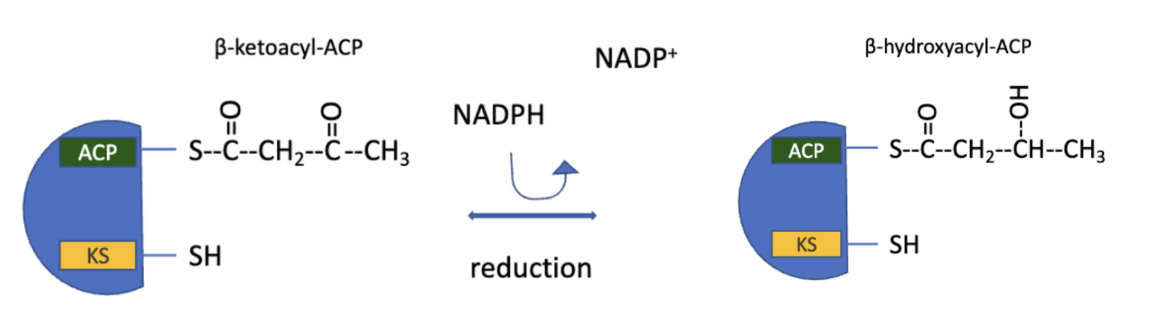
FAS: Step 3 Dehydration
the elements of water are removed from C2 and C3 of D-β-hydroxybutyryl-ACP to yield a double bond in trans-∆2-butenoyl-ACP
catalyzed by β-hydroxyacyl-ACP dehydratase
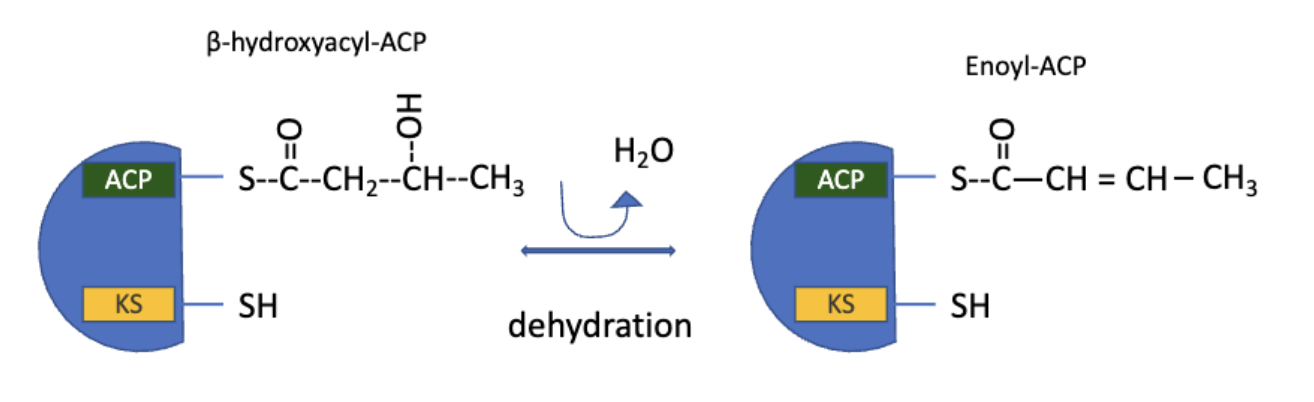
FAS: Step 4 Reduction
the double bond of trans-∆2-butenoyl-ACP is reduced (saturated) to form butyryl-ACP
catalyzed by enoyl-ACP reductase
electron donor: NADPH
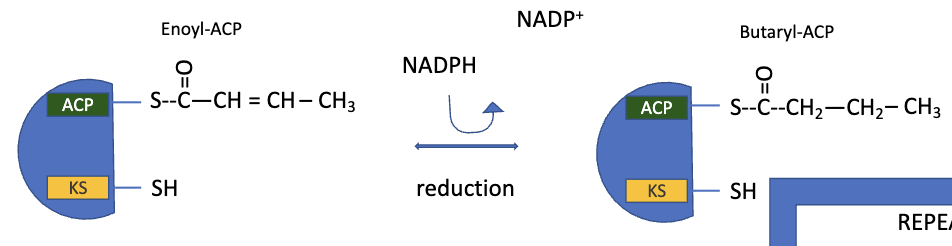
FAS: Step 5 Translocation
production of the 4-carbon, saturated fatty acyl-ACP marks completion of one pass thru the fatty acid synthase complex
the butyryl group is transferred from teh phosphopantetheine–SH group of ACP to the Cys–SH group of β-ketoacyl-ACP synthase (which initially bore the acetyl group)
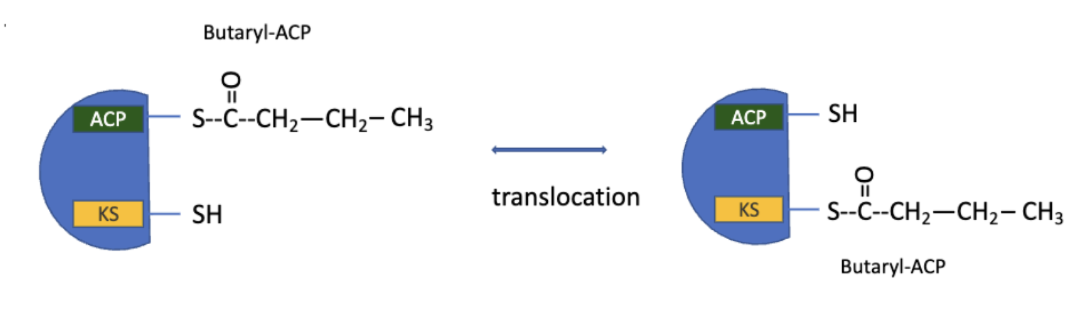
FAS: Step 6 Repeat
to start the next cycle of 4 rxns that lengthens the chain by two more carbons, another malonyl group is linked to the now unoccupied phosphopantetheine–SH group of ACP
condesation occurs as the butyryl group, acting like the acetyl group in the first cycle, is linked to two carbons of the malonyl-ACP group with concurrent loss of CO2
Overall Reaction of FAS (Synthesis of Palmitate)
Formation of 7 malonyl-CoA molecules
7Acetyl-CoA + 7CO2 + 7ATP → 7 malonyl-CoA +7ADP +7Pi
7 cycles of condensation and reduction
Acetyl-CoA + 7malonyl-CoA+14NADPH +14H+ → palmitate + 7CO2 + 8CoA +14NADP+ + 6H2O
Overall process:
8 Acetyl-CoA + 7ATP +14NADPH +14H+ → palmitate +8CoA+7ADP +7Pi +14NADP+ + 6H2O
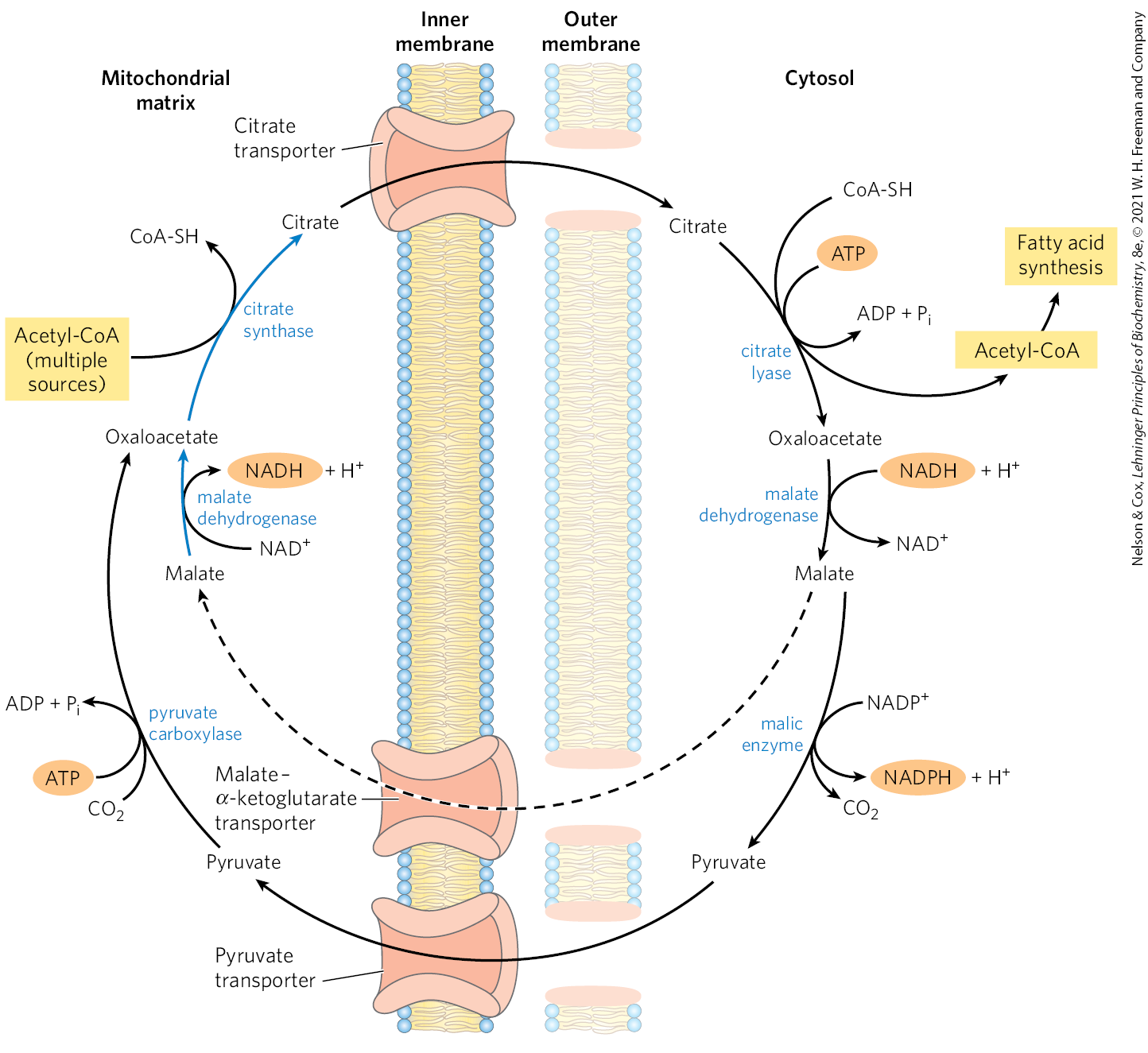
Citrate Transporter: Moving Acetyl Units out of Matrix
intramitochondrial acetyl-CoA reacts with oxaloacetate to form citrate, catalyzed by citrate synthase (also an enzyme in TCA cycle)
acetyl-CoA made from pyruvate dehydrogenase or β-oxidation
citrate then passes thru the IMM on the citrate transporter
in the cytosol, citrate cleavage by citrate lyase regenerates acetyl-CoA and oxaloacetate in an ATP dependent reaction (for FA synthesis)
oxalocaetate can’t return to the matrix, as there’s no oxaloacetate transporter
Citrate Transporter: Malate Fates
Matrix Fate
cytosolic malate dehydrogenase reduces the oxaloacetate to malate, which can return to the mitochondrial matrix on the malate-⍺-ketoglutarate transporter, in exchange for citrate
in the matrix, malate is reoxidized to oxaloacetate to complete the shuttle
OR
Cytosol Fate
be converted in pyruvate by malic enyme which is transported into the matrix by the pyruvate transporter and converted into oxaloacetate
this pathway produces NADPH, which is required for FA synthesis and is one of the major ways in which the cell generates this electron acceptor
Pyruvate Transporter
Transports pyruvate into the matrix where it is converted to oxaloacetate by pyruvate carboxylase
Citrate Malate Shuttle Figure
in the resulting cycle, two ATP molecules are consumed (by citrate lyase and pyruvate carboxylase) for every molecule of acetyl-CoA delivered to FA synthesis
citrate lyase (cytosol): cleaves citrate → acetyl-CoA + oxaloacetate (1ATP)
pyruvate carboxylase (matrix): regenerates oxaloacetate from pyruvate after the cycle runs (1ATP)
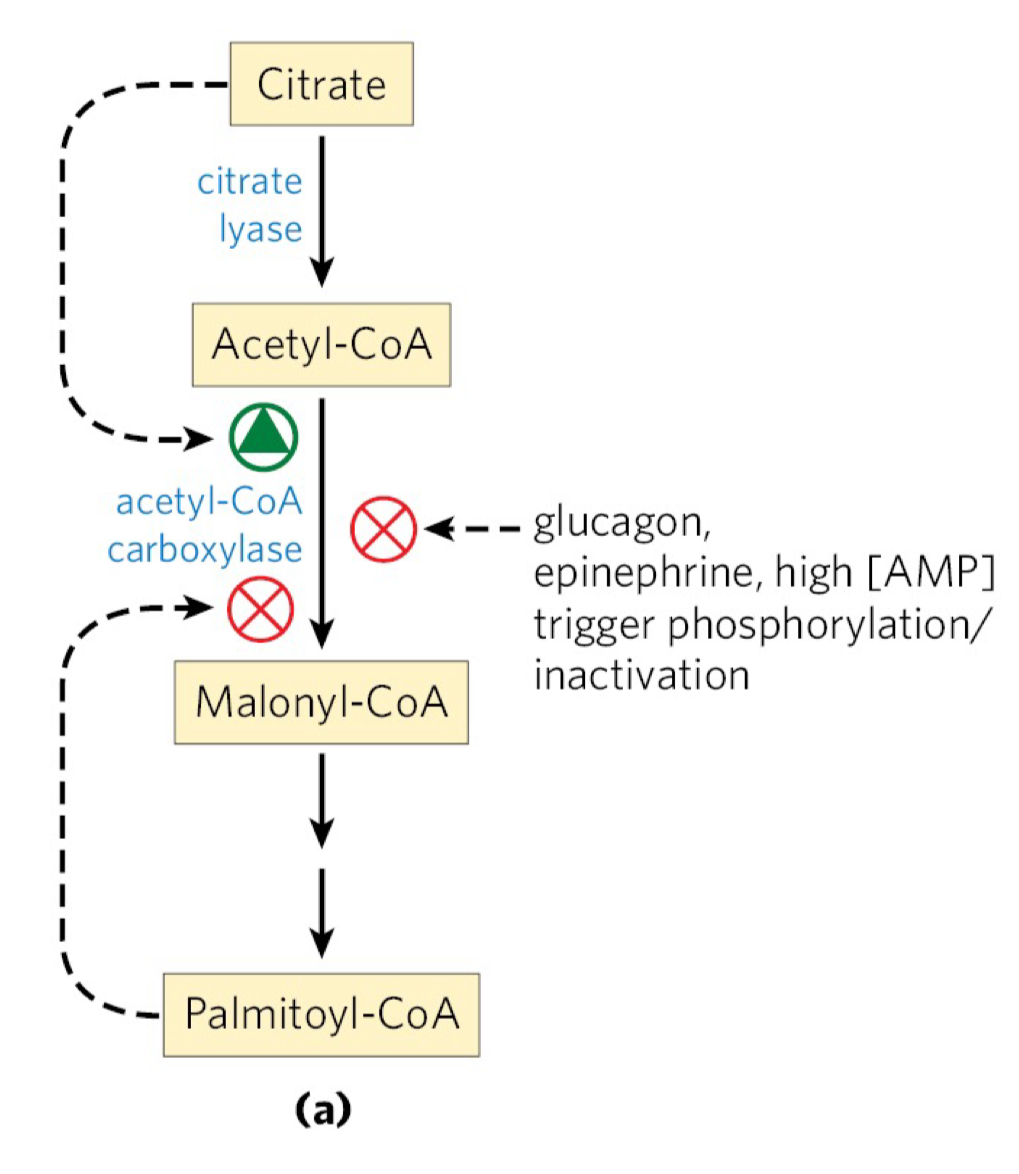
Acetyl-CoA Carboxylase (ACC) Regulation
we want to synthesize fatty acids when there is an abundance of energy and Acetyl-CoA available
we want to reduce/restrict synthesis when there is not
ACC, which synthesizes malonyl-CoA (substrate of FA synthesis), is inhibited by glucagon and by high levels of palmitoyl-CoA
glucagon: secreted when blood glucose levels are low in the fasting state
palmitoyl-CoA: ultimate product of FAS, negative feedback regulator
the rxn catalyzed by ACC is the rate-limiting step in FA synthesis
when concentration of mitochondrial acetyl-CoA and ATP increase, citrate is transported out of the mitochondria
it then becomes the precursor of cytosolic acetyl-CoA and an allosteric signal of the activation of ACC
Acetyl-CoA Carboxylase (ACC): Phosphorylation Regulation
Dephosphorylation: ON
Phosphorylation: OFF
ACC and CAT1 Regulation
if FAS and β oxidation proceeded simultaneously, energy would be wasted
because ATP and NADPH are spent to make fatty acids only to immediately oxidize them back into acetyl-CoA.
high blood glucose dephosphorylates ACC, making it more active
the product, malonyl-CoA inhibits CAT1, restricting the amount of fatty acyl-CoA that can enter the mitochondria for oxidative breakdown (β oxidation)
in the fasting state, ACC is inactive and malonyl-CoA is not being synthesized, β-oxidation is favored