Commensal Bacteria vs Pathogens
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
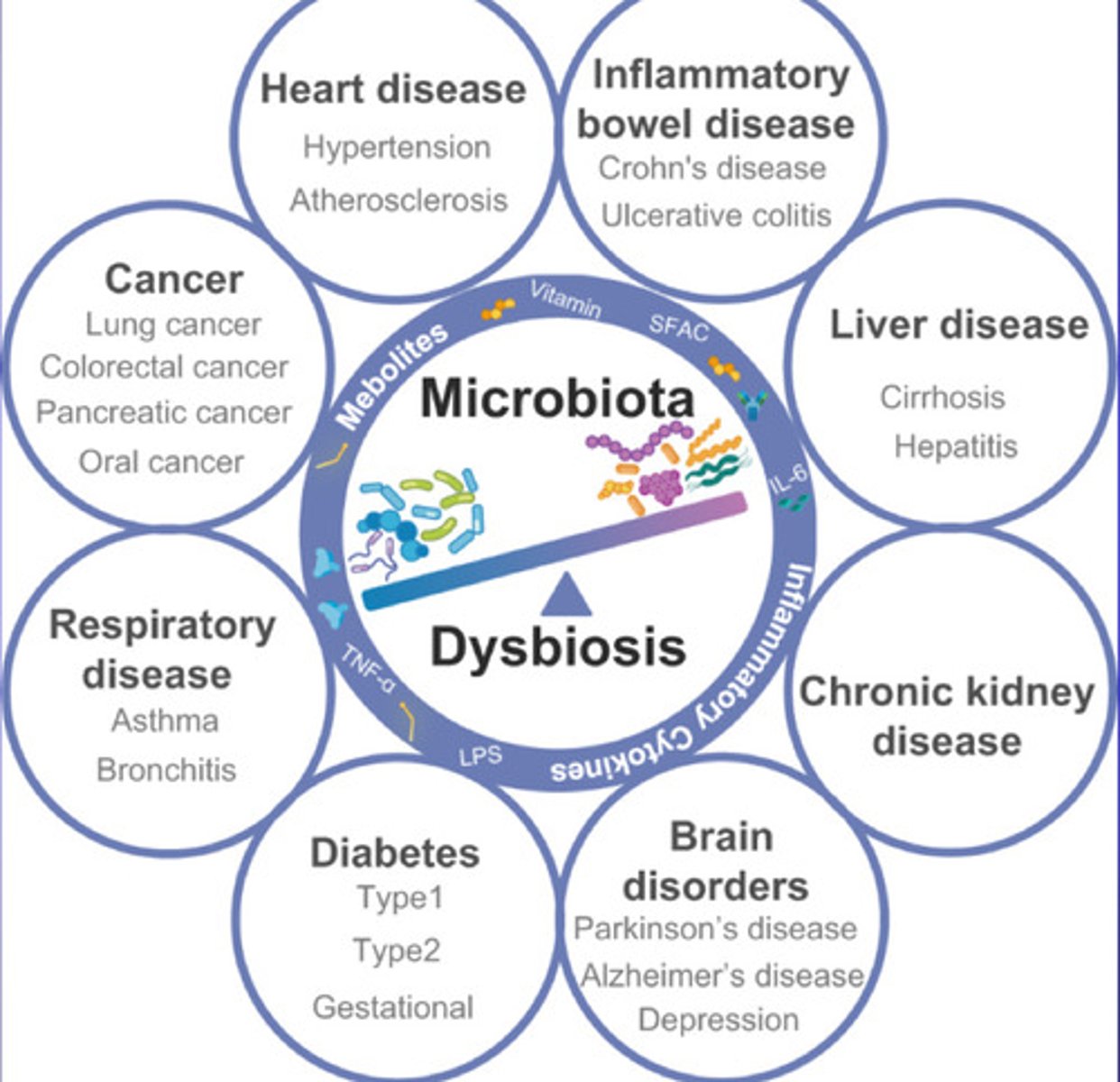
What is Microbiota Dysbiosis?
an imbalance in the microorganisms that live in the gut. This imbalance can occur when there is a loss of beneficial bacteria, an overgrowth of harmful bacteria, or a loss of overall microbial diversity
associated with conditions named on the diagram
what is included in the gut?
another name for the gastrointestinal (GI) tract, which is the system of organs that digest food and absorb nutrients:
The gut includes the stomach, intestines, and colon, and is responsible for: Digesting food, Absorbing nutrients, and Excreting waste
list ways we can treat Microbiota dysbiosis?
-Diet, pre biotics (Non-digestible food ingredients that can stimulate the growth of beneficial bacteria in the colon), post biotics
-Probiotics
-Live biotherapeutic product
-engineered bacterial
-Probiotics
-Faecal microbiota transplantation
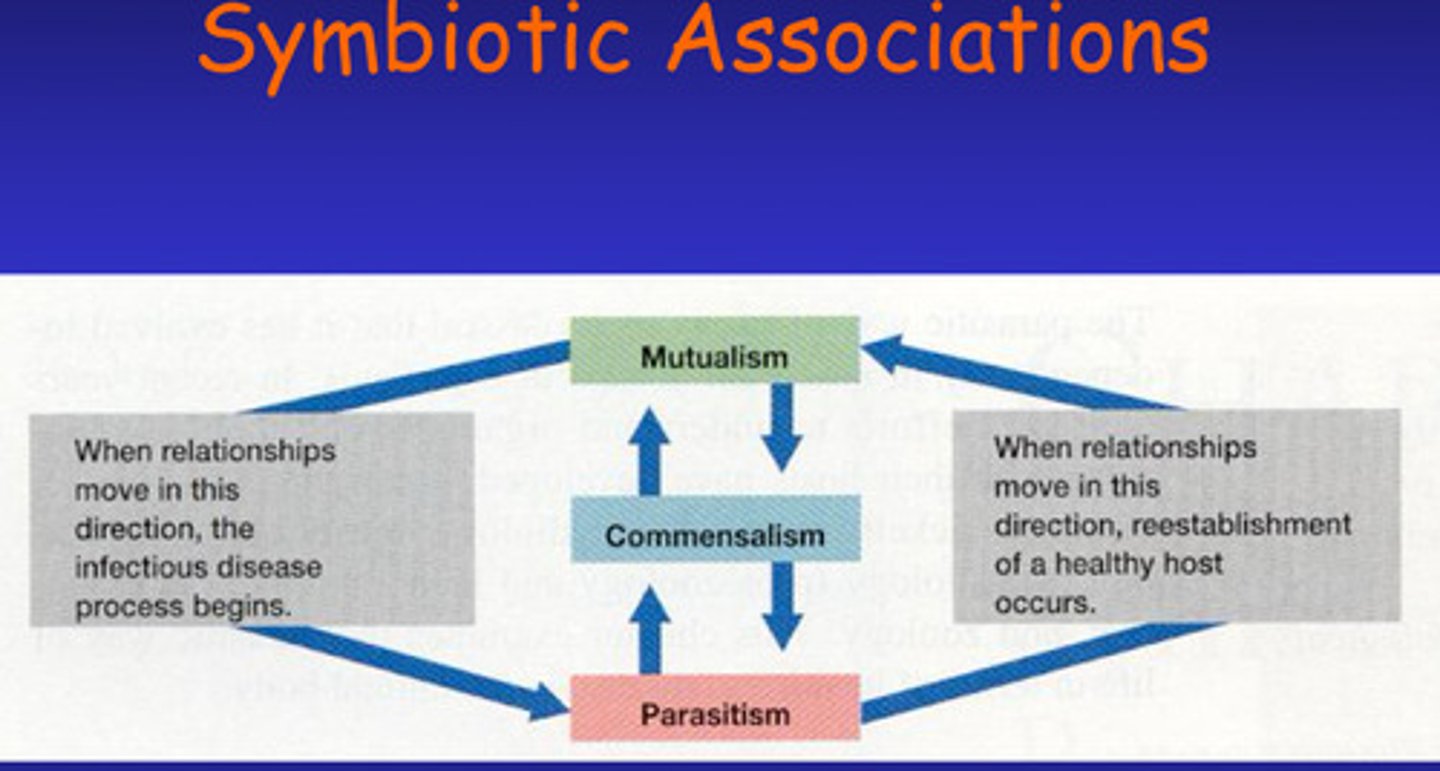
What is symbiotic association and what are the 3 types of this?
a close, long-term relationship between two or more different species of organisms
3 Types:
Commensalism - relationship in which one symbiant called the commensal benefits while the host is neither harmed nor helped - Due to the spacial proximity of the two, the commensal obtains nutrients ingested or captured by the host and the host may also provide shelter to the commensal which can live either on or in the host.
-Commensal can survive without the host
Mutualism:
-Some reciprocal benefit occurs to both partners - the mutualist and the host are metabolically dependent on each other e.g. lichens on trees
Parasitism:
-If a symbiont either harms or lives at the expense of another organism
-Linked to infectious diseases
Note:
These aren't static - the type an organism can change depending on the conditions etc. Commensals becoming parasites, and when parasites invade the body - infection (disease when it starts causing symptoms)
-Persistence in a body sight without causing disease is called colonisation (not infection)
-INFECTION DOES NOT ALWAYS LEAD TO DISEASE, if host defence mechanisms are adequate a disease causing organism can cause infection with no symptoms (Asymptomatic carriage)
-One persons colonisation is someone elses infection
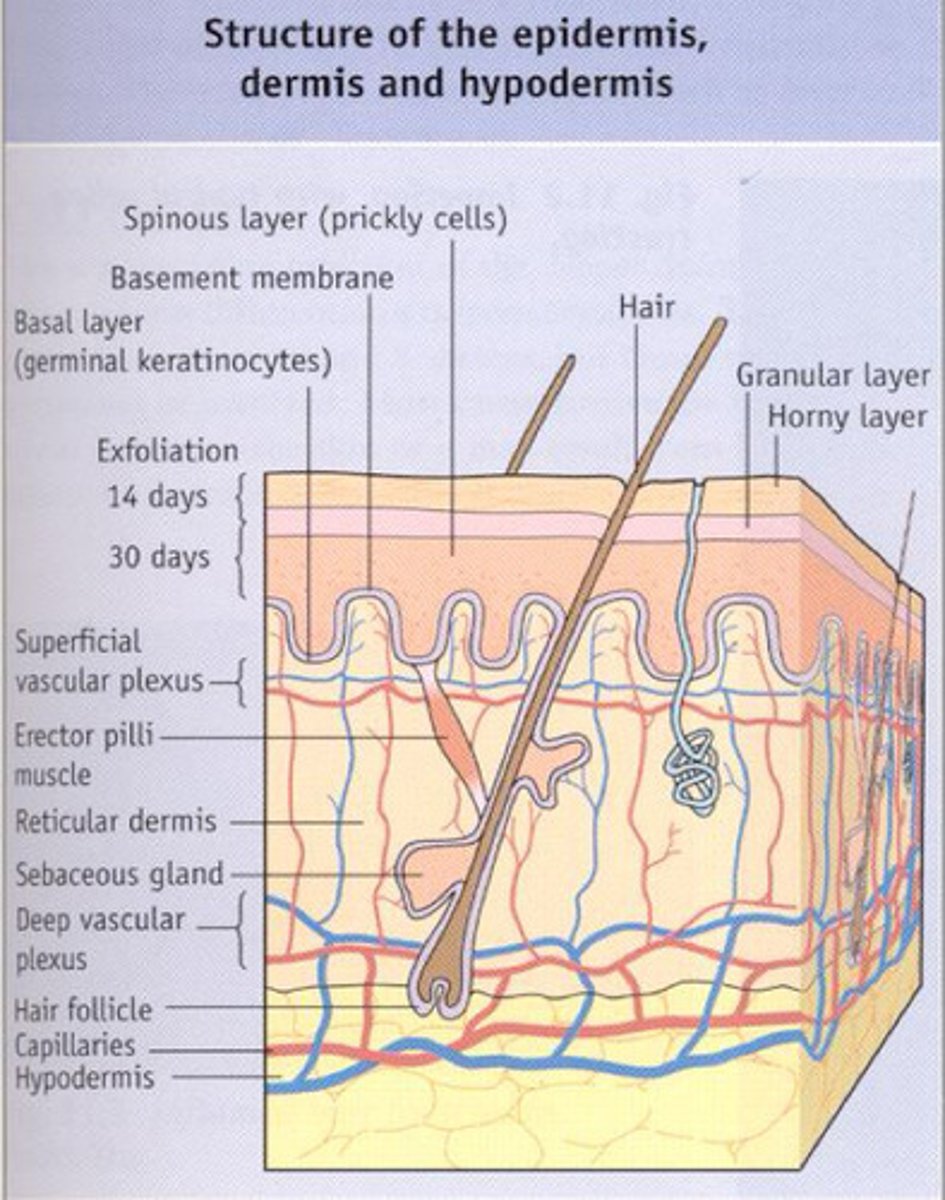
Elaborate on the microbiome of the skin - name 2 beneficial bacteria that is provided here
-The epidermis is not a favourable environment for microbes - Periodic drying causes many resident microbiota to become dormant
(but certain areas e.g. scalp, ears, palm, genital urinary and anal regions are moist enough to support commensals)
-Epidermis has acidic pH due to oil and sweat secretions - sweat has sodium chloride giving the epidermis osmotic pressure that helps regulate the water balance on the skin surface and prevents excessive water loss, while also creating an environment that is hostile to many harmful microbes. In combination with the production of inhibitory compounds e.g. lysozyme, the skin controls the colonisation, overgrowth and infection from commensal organisms.
-Most skin bacteria are associated with glands that provide nutrients e.g. sebaceous glands providing
-A types of beneficial skin bacteria Staphylococcus epidermidis (italics after you write the names)
-Most beneficial type of skin bacteria produced is gram positive anaerobe Propionibacterium acnes
(Remember the names of the bacteria in italics )
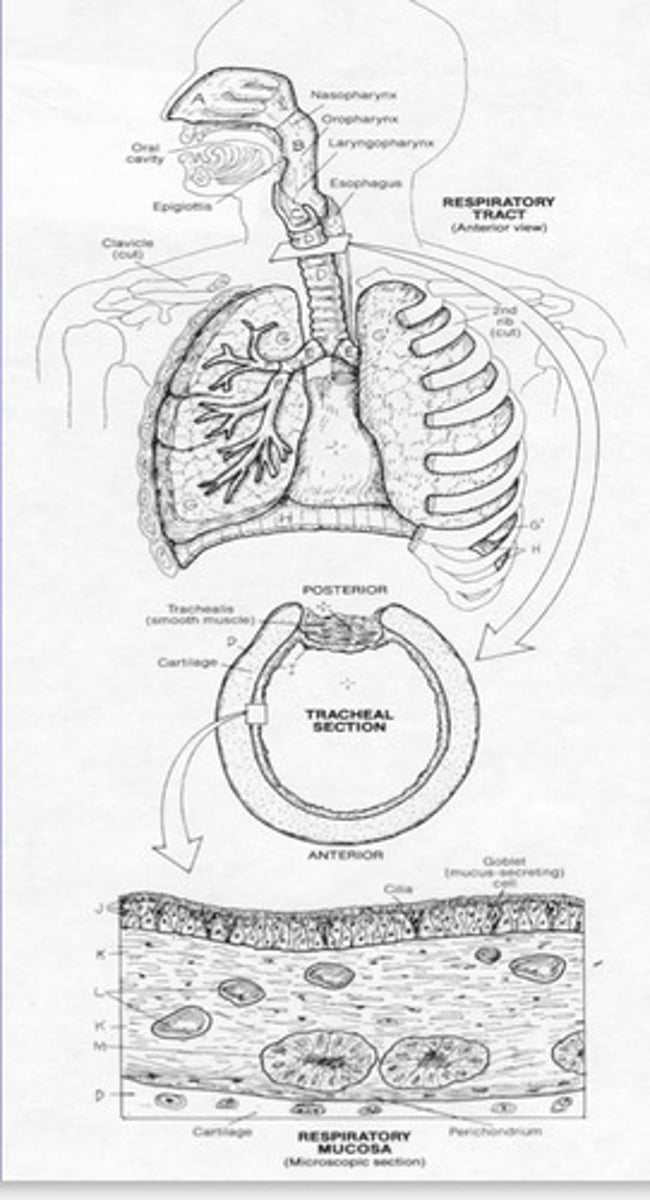
Elaborate on the Microbiome of the Respiratory Tract
-Staphylococcus epidermidis and Staphylococcus aureus are predominant in the nose in equal numbers as those on the skin
-Nasopharynx contains small numbers of staphylococcus pneumoniae, Neisseria meningitidis and Haemophilus influenza. ALL PATHOGENIC MICROORGANISMS.
-But usually strains that are found in the nasopharynx as part of the normal microbiome lack capsule virulence factors (Virulence factors are the molecules that assist the bacterium colonize the host at the cellular level) = no potential to cause disease in the person that carries them as commensals
-Most important bacteria found in the Oropharynx are the alpha hemolytic streptococci, large numbers of Diphtheroids and small gram negative cocci related to Neisseria.
-The Respiratory tract doesn't usually have a microbiome because microorganisms are removed by a continuous stream of mucus generated by ciliated epithelia. There's also phagocytic action in the lung.
-Lysosome present in nasal mucus also has an antibacterial effect
-The normal microbiome of the oral cavity contains organisms capable of resisting mechanical removal by adhering to surfaces e.g. gums and teeth and tongue.
-Oral cavity is colonised within hours of birth - initially the microbiota consists of Genera e.g. actinomyces, Streptococcus, lactobacillus, Neisseria and yeast
-As teeth appear, the dominant organisms are anaerobic due to colonisation by bacteria e.g. streptococci which are also attached to the buccal and gingivitis epithelial surfaces and colonise the saliva
-Bacteria adds to the normal dental plaque, caries (a disease that occurs when bacteria in your mouth produce acids that break down your teeth), gingivitis (the earliest stage of gum disease) and periodontal disease.
-Overgrown commensal organisms can become pathogenic
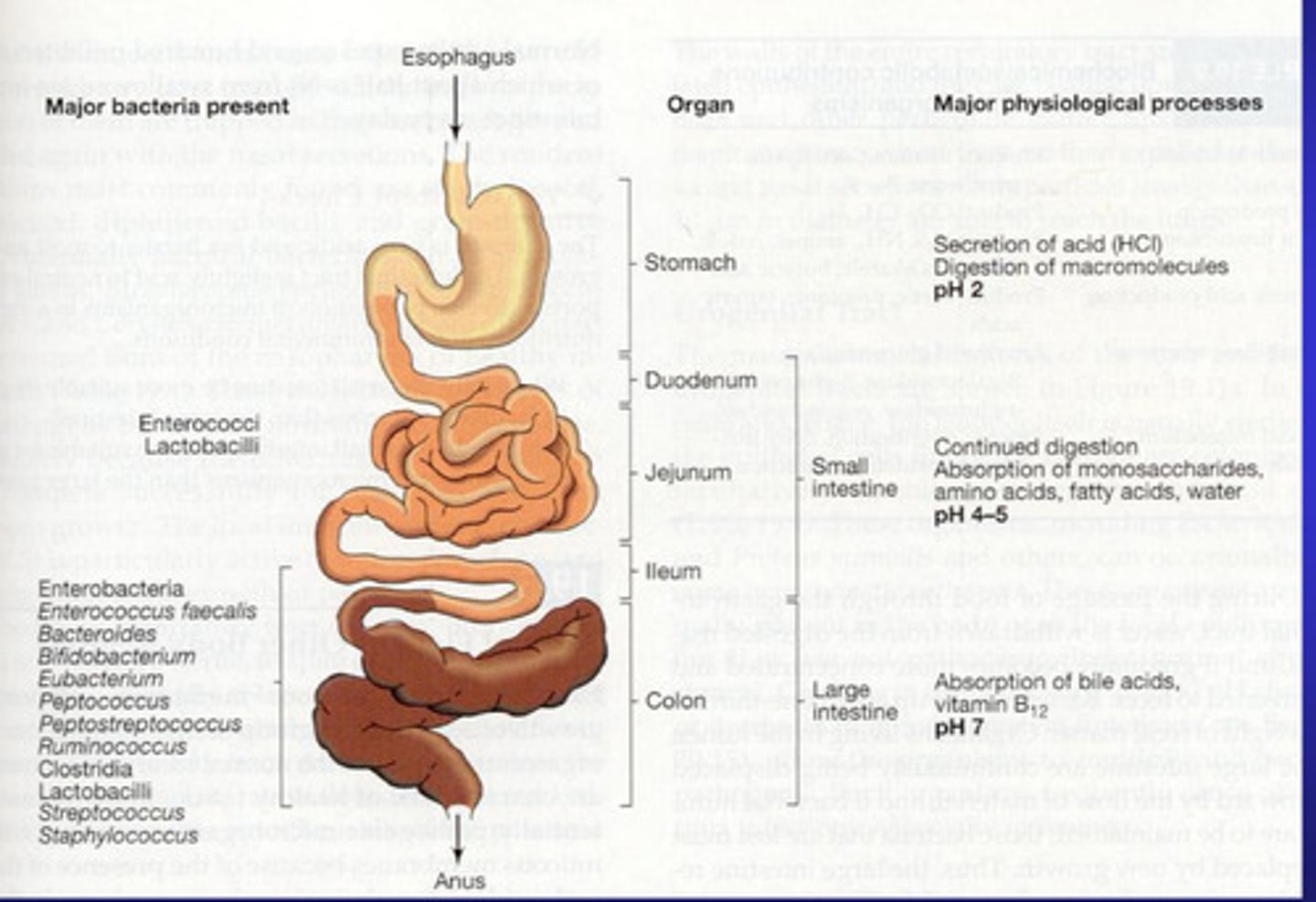
Elaborate on the Microbiome of the GI Tract - Stomach, Small intestine and Colon
Stomach
-The stomach contains many microorganism
-Stomach is very acidic which kills many microorganims - those that can survive (not many) include genera such as lactobacillus, Peptostreptococcus, and yeast - such as candida.
-Number of bacteria usually increases after a meal but the number drops after acidic nature of the stomach carries out its effect.
- Mycobacteria can survive passage through the stomach
-Changes in the gastric microbiota occur when there is an increase in pH e.g. intestinal obstruction = duodenal reflux, = microbiota of the stomach will reflect the oropharynx. Helicobacter pylori will be an exception in the stomach.
Small Intestine:
-Split into duodenum, jejunum and ileum
-Duodenum has few microorganism due to combined influence of the acidic gastric juices and the action of bile and pancreatic secretions
-The jejunum Gram positive rods and cocci dominate
-In the ileum the microbiota mimic the colon. Gram negative bacteria and members of the family Enterobacteriaceae dominate in these alkali environments.
Colon:
-Has largest microbial community in the body
-Microbiota consists of mainly of anaerobic gram negative non spore forming bacteria as well as bacteria, yeast and protozoa may occur as harmless commensals.
-Normal microbiota of GI tract is influenced by diet e.g. the Prescence/lack of bifido-bacterium and lactobacillus is dependent on feeding of new born infants
-Antibiotics effect Prescence of bacteria
-Normal microbiome has functions against infections
Elaborate on the Microbiome of the GU (Genital Urinary) Tract
-The upper GU tract - kidneys ureter and bladder are usually very sterile and only a few genera are present in the distal portions (leading out of the body) in the urethra e.g. Staphylococcus epidermidis and enterococcus faecalis
-The female GU tract has complex microbiota due to its large Surface area - changes with the menstrual cycle
-The major microorganisms are the acidic tolerant lactobacilli that ferments glycogen secreted by the vaginal epithelium which produces lactic acid which maintains the pH of the vagina to around pH 4.5-4.6
What is a virulence factor?
characteristic or structure that contributes to the ability of a microbe to cause disease
What is Koch's postulates and why is it used?
Why may it not always be possible to prove Koch's postulates?
-Used to prove a pathogen (organism) is responsible for a particular disease
▪ Organism must be found in all host’s with disease
▪ Organism must be isolated in pure culture
▪ Organism should produce the same disease when inoculated into a healthy host
▪ Organism should be re-isolated in pure culture
Drawbacks of Kochs Postulates:
-Virulence is purely microbial and is independent of the host
-Many organisms can't be cultured on standard lab media (although 16 S ribosomal RNA sequencing has overcome this)
-Unethical to reinfect a susceptible host
-Virulence can be lost on culture when re-isolation
What is the molecular version of Koch's Postulates and what standards need to be met?
-Focus has shifted now towards identifying the genes/products that give pathogenic potential
▪ Genes (or their products) have pathogenic potential
Standards:
▪ Gene should be found in all pathogenic strains but not in avirulent strains
▪ Disruption of the gene should reduce virulence
▪ Avirulent strains can be transformed into virulent strains by cloning the gene ▪ The gene must be expressed during the infectious process
▪ The gene product should elicit an immune response
How are pathogens transmitted?
-Indirect airborne
-Direct Airborne
-Contact
-Via hands or objects
-Dust borne
How do pathogens adhere to the surface of a new host?
-On the pathogen as they have fimbria structures e.g. Pillai - short polypeptide chains that come out of the surface of bacteria that allow the organism to attach to the epithelial surface - they are constantly being reformed as they are easily broken
-Transient interaction usually as they break off quite easily because of the immune response to them - once antibodies have bound to pillai tips = no adhesion.
-Some microorganisms have cell surface proteins = tighter binding after initial binding of pillai (a fimbria adhesions)
-Another adherence mechanism is Formation of biofilms, where microorganisms form dense multi-organism layers on surfaces, first layer attaches directly to the substrata and other layers are attached to basal layers by polysaccharide matrix- certain organisms will produce their glycocalyx (mucus slime) that allows them to attach and grow in a surface and then move away from the surface and can actually go against gravity also.
What do Microorganisms do once they have adhered to the host?
-Once colonisation has occurred, microorganism produces enzymes that breakdown the epithelial surface and attack the immune response of the host .
-Once the organism has penetrated the cell surface it wants to go to places with more nutrients
- It stays in the one area (loci of infections) but eventually the nutrients will run out and it will need to disseminate through the host:
-Can be done by passing freely through blood and lymph (but will be exposed to immune response) so they opt to invade cells and cause them to rearrange their cytoskeletons (actin rearrangement) e.g. Candida albicans (a yeast)
How do microorganisms Grow and multiply?
▪ Free iron is limiting in natural environments
▪ They secrete Siderophores (catechols/hydroxamates) chelate free iron - irons will absorb up across its membranes
▪ Exotoxins (proteins that secrete while it is a vegetative cell) can release bound iron from cells - These toxins breaks down our tissues releasing iron from the cells
How do microorganisms (pathogen) evade the immune response ?
-Necessity !
-Producing Glycocalyx structure
-The complement pathway - series of proteins that attack bacteria - Organism will try to inhibit this cascade of proteins
-Microorganisms also have anti-phagocytic strategies
Mock question: In July 2015, a report was released indicating the Gram-negative bacterium Pseudomonas aeruginosa was found on hospital sinks 10 years after the initial outbreak in a neonatal intensive care unit. P. aeruginosa usually causes localized ear and eye infections but can cause pneumonia or septicaemia in vulnerable individuals like newborn babies. Explain how the current discovery of the presence of this reported P. aeruginosa could lead to a recurrence of nosocomial (a disease originating in hospital) disease.
P. aeruginosa persistence: This bacterium is known for its ability to form biofilms on surfaces, including hospital sinks and plumbing systems. Biofilms provide a protective environment for the bacteria, allowing them to survive in harsh conditions for long periods, making it difficult to completely eradicate them through standard cleaning procedures. These biofilms can act as a reservoir of infection.
Resistant nature: P. aeruginosa is naturally resistant to many antibiotics and disinfectants. This makes it particularly dangerous in a hospital setting where patients, especially vulnerable populations like newborns in NICUs, can be exposed. Once the bacterium is established in the hospital environment, it can be challenging to remove and prevent the spread.
Nosocomial transmission: Hospital sinks are frequently used by healthcare workers for handwashing or other procedures. The water droplets or contaminated surfaces can act as a vehicle for P. aeruginosa to spread to medical equipment or hands, leading to cross-contamination and increasing the risk of infection in patients, particularly those with weakened immune systems.
Recurrent outbreaks: The presence of P. aeruginosa in hospital infrastructure, like sinks and plumbing, can lead to recurrent outbreaks over time, especially if infection control practices are not sufficiently robust. Once reintroduced into a clinical setting, it can infect patients again, resulting in increased morbidity and potentially mortality among high-risk groups.
Mock question: A microbiologist has identified a new Gram-negative pathogen that causes liver disease in rats. They suspect that the bacterium’s fimbriae are a virulence factor. Describe how molecular Koch’s postulates could be used to test this hypothesis.
Identify the gene responsible for fimbriae production: First, identify the gene or genes in the bacterium that are responsible for the production of fimbriae, the hair-like structures that might help the bacteria adhere to host cells.
Inactivate the fimbriae gene: Using genetic techniques such as gene knockout or CRISPR, the researcher would create a mutant strain of the bacterium that lacks the fimbriae by disabling the specific gene.
Test the virulence of the mutant strain: The microbiologist would then infect rats with both the wild-type (normal) bacterium and the mutant strain lacking fimbriae. If the mutant strain shows reduced virulence (i.e., causes less severe liver disease or fails to infect the rats), it supports the hypothesis that fimbriae contribute to virulence.
Restore the gene in the mutant strain: To confirm the role of fimbriae, the next step would be to restore the fimbriae-producing gene in the mutant strain (this process is called complementation). If this restored strain regains virulence, it provides strong evidence that fimbriae are indeed a virulence factor.
Demonstrate the gene’s expression during infection: Finally, it would be important to show that the fimbriae are expressed during infection in the rats. This can be done by detecting the presence of fimbriae or the relevant gene products (proteins) in infected liver tissues.