Kp + partial pressure
0.0(0)
0.0(0)
Card Sorting
1/8
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
9 Terms
1
New cards
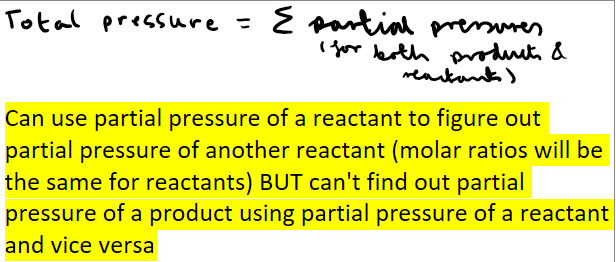
How do you calculate total pressure from partial pressure?
2
New cards
How do you calculate mole fraction of gas in a gas mixture?

3
New cards
How do you calculate partial pressure of a gas?
Measured in kPa
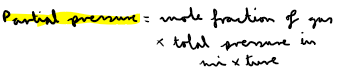
4
New cards
How do you calculate Kp in homogeneous equilibria?
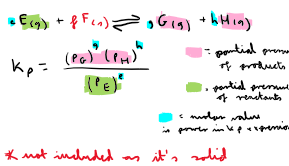
5
New cards
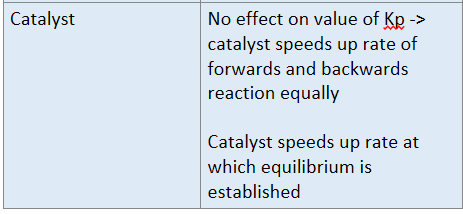
What are factors that affect Kp?
Temperature
Pressure
Catalyst
6
New cards
How does position of equilibrium affect Kp?
MAIN PRINCIPLE-
Equilibrium shifts to right: Kp value increases
Equilibrium shifts to left Kp value decreases
7
New cards
How does temperature affect Kp?

8
New cards
How does pressure affect Kp?

9
New cards
How does a catalyst affect Kp?