Chemistry last unit test (previously Chemistry solutions concept check)
1/40
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
Solubility
Ability of a substance (solute) to dissolve in another substance (solvent)
What must happen for a solute to dissolve in a solvent?
There must be attractive forces between the solute and solvent
Solute solvent forces>= Solute-solute IMFs
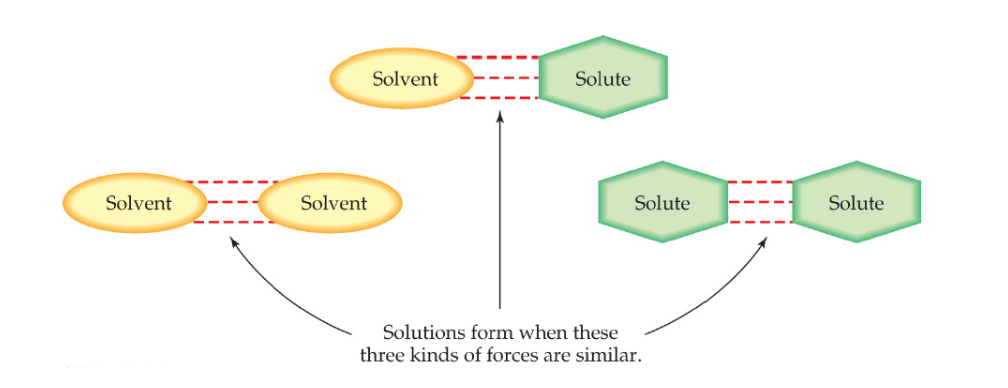
Solution
A mixture containing atoms, ions, or molecules fully dissolved
Colloid
A mixture containing mid-sized particles that do not settle out
Suspension
A mixture containing particles that settle out if undisturbed.
Tyndall effect
If you shine light through a solution, the light will not be seen through the container. If you shine light through a colloid, you will get a cloudy beam of light. You can see light through a colloid because the particles in a colloid are bigger, which scatters the light, making the light visible to us.
What does “like dissolves like” mean
Polar solvents dissolve polar solutes and non polar solvents dissolve polar solutes. In a polar solution, the polar solvent is charged and can take the opposite charge that the solute has and rip that charge off the solute so that the solvent is now bonded to an oppositely charged particle solute. The bond forming between solvent and solute allows the particles to dissolve.
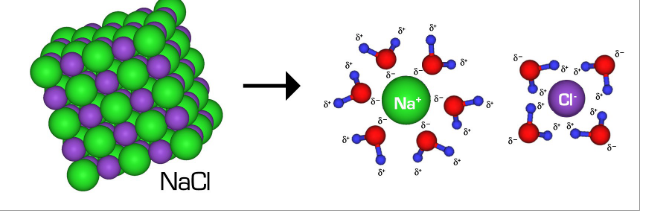
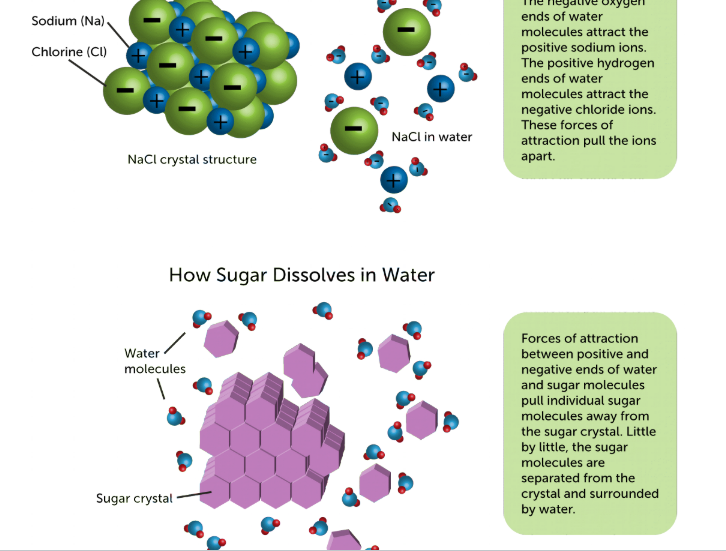
Ionic vs covalent compounds dissolving
In an ionic compound, each ion is taken off, but in a covalent compound, the whole compound attaches to the molecule of the solvent
Unsaturated solution
More solute dissolves
Saturated solution
No more solute dissolves
Supersaturated solution
Becomes unstable, crystal form. In a supersaturated solution all of these solute remains dissolved (no visible crystals). If a supersaturated has been bumped or shaken, the disturbance causes some solute to crystallize on the bottom, making the solution saturated after the disturbance.
Solubility and temperature
For solids and liquids, as temp increases, solubility increases. For gasses, as temp increases, solubility decreases.
Dissolving a solute involves:
breaking of bonds/IMFs between solute particles (energy absorbed)
Breaking of bonds/IMFs between solvent particles (energy absorbed)
Forming of new bonds/IMFs between the solute and solvent (generally energy is released
Molarity
Moles of solute/Liters of solution. M=n/V. The denominator is Liters of solutions, not just the Liters of solvent. The denominator takes into consideration the volumes of both the solute and the solvent.
Dilution equation
M1V1=M2V2
During dilution, the moles of solute do not change, only the total volume of solution increases, which causes the molarity to decrease
Pressure and solubility
Liquids and solids: no change
Gasses: increase in pressure=increase in solubility, decrease in pressure=decrease in solubility
Factors that affect dissolving
Surface area
dissolution takes place only at the surface or each solute particles. When the total surface area of the solute particles is increased, the solute dissolves more rapidly
The more the surface area that is exposed to the outside the faster it will dissolve (think of a sugar cube, only the outside of the cube (the surface area) will dissolve immediately, whereas in sugar pieces the surface area of each solute particle is increased, and the solute dissolves faster because the solvent can break it down more easily. Creating more surface area is done by making smaller pieces.
Agitation (stirring, shaking)
Solid/liquid solutes: agitation allows the solute to dissolve faster
Gaseous solutes: Agitation causes gases to dissolve more slowly, agitation causes gases to escape from the solution
Amount of solute already dissolved
When you have very little solute in the solution, dissolving takes place quickly. When you have a lot of solute in the solution, dissolving takes place more slowly.
Acids vs bases Arrhenius definition
acids are hydrogen-containing compounds that dissociate in water to give H+ ions or protons, while bases are hydroxide compounds that dissociate to give OH– ions.
Acids vs bases bronsted-lowry definition
A Brønsted-Lowry acid is any species that can donate a proton, H+, and a base is any species that can accept a proton
Why can we write a proton and H+ interchangeably?
Protons and H+ ions both hold a charge of +1
How to find an acid in a reaction
Look for something with an H, if you look at the product side and see it has a more negative charge it is an acid because it lost a a positive H+ ion, making the charge more negative.
How to find a base in a reaciton
look for something that gains a positive charge/becomes less negative, because it is receiving the positive H+ ion
Properties of acids
Taste sour or tart
Corrosive
pH<7 (but a higher pOH)
Release H+ ions
Turn litmus paper red
Examples: Lemon juice, vinegar, soda
properties of bases
Taste bitter
Feel slippery
Corrosive
pH>7 (but a lower pOH)
Release OH- ions
Turn litmus paper blue
Examples: Drain cleaner, soap, household ammonia
Electrolytes vs. Non-electrolytes
electrolyte: A compound that conducts an electric current when it is in an aqueous solution or melted.
non-electrolyte: A compound that does not conduct an electric current in either aqueous solution or in the molten state
Strong acids and bases
Ionizes completely
conducts electricity well (strong electrolytes)
do not undergo reversible reacitons
Weak acids and bases
only ionize partially in water
weak electrolytes (if a light bulb was placed in this solution, it would light up but it would be dim)
reversible reactions
Strength is NOT dependent on concentration of the solution
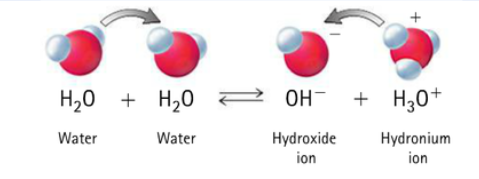
Water and its reaction with itself
Water behaves as both an acid and a base
In a beaker of water, a small portion of water molecules will transfer H+ to another water. This produces H3O+ (hydronium) and OH-.
Note: H+ ions usually do not float around freely; they usually attach to water and form H3O+. For simplicity, we often just write them as H+
H3O+ (or H+) ions would make the solution acidic. OH- ions would make the solution basic.
There are equal amounts of H3O+ and OH-, so they “cancel” each other and the beaker of water is neutral (pH 7).
What happens when acids are added to water?
When acids are added to the water, the amount of H3O+ (or H+) increases (and OH- is now much smaller)
Acids: [H3O+] > [OH-]
pH < 7
There is still OH- present because the reaction water has with itself still happens in the background
![<p>When acids are added to the water, the amount of H3O+ (or H+) increases (and OH- is now much smaller)</p><p>Acids: [H3O+] > [OH-]</p><p>pH < 7</p><p>There is still OH- present because the reaction water has with itself still happens in the background</p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/d2967067-e82a-4cba-a73e-98046c665217.png)
The pH scale
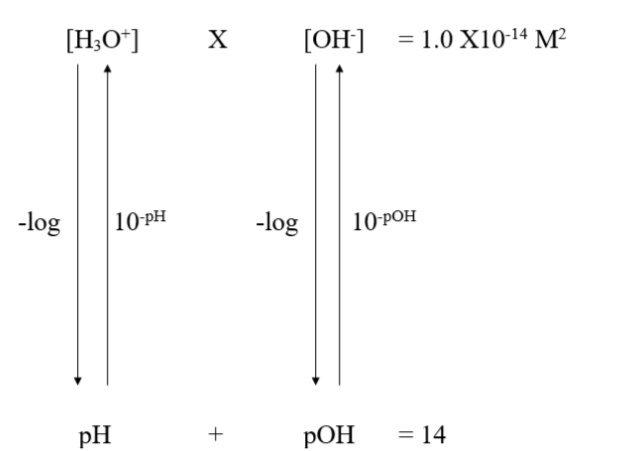
The term pH means “powers of hydrogen” and measures the concentration of H+ (or H3O+) ions in the solution.
The concentration of H3O+ (or H+) in a solution is usually small and expressed in scientific notation.
Example: In pure water, the molarity for both H3O+ and OH- is 1.0 x 10-7 M
How to find the pH of a soution
The pH of a solution can be found by using the equation
pH = -log [H+] OR pH = -log [H3O+]
Note: [H+ ] means molarity
pH values are the power of 10 found in the H+ concentration (without the negative sign)
As the [H+] decreases, what happens to pH? Is the solution becoming basic or acidic?
the solution is becoming more basic because the molarity of pH is getting smaller, meaning there is less H+ which makes for a more basic solution
If you lower the pH from 4 to 2, how many times more acidic is it becoming?
100 times more acidic
What is pOH?
pOH measures the concentration of OH- in a solution
pOH = -log [OH-]
The pOH scale runs in the opposite direction of the pH scale
A low pH (high [H+]) corresponds to a high pOH (low [H+])
pH + pOH = 14
On both scales, the lower the number, the higher the amount there is (lower pH, more acidic, lower pOH, more basic)
![<ul><li><p><span>pOH measures the concentration of OH- in a solution</span></p></li><li><p><span>pOH = -log [OH-]</span></p></li><li><p><span>The pOH scale runs in the opposite direction of the pH scale</span></p></li><li><p><span>A low pH (high [H+]) corresponds to a high pOH (low [H+])</span></p></li><li><p><span><mark data-color="purple">pH + pOH = 14</mark></span></p></li><li><p><span><mark data-color="purple">On both scales, the lower the number, the higher the amount there is (lower pH, more acidic, lower pOH, more basic)</mark></span></p></li></ul>](https://knowt-user-attachments.s3.amazonaws.com/d1f30d73-d92b-4320-8026-3e38d6194439.png)
Roadmap for Solving pH & pOH Problems
just a picture lol
What is the [H+] and [OH-] for 13pH?
[H+]=1.0×10^-13 [OH-]=1.0×10^-1
high pH so basic solution
What is the [H+] and [OH-] for pOH 8?
[OH-]=1.0×10^-8 [H+]=1.0×10^-6
slightly high pOH so acidic solution
Neutralization reactions
strong acids and bases react to produce water and salts
Ex: HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
Because these reactions produce neutral solutions (pH = 7), they are called neutralization reactions. Neutralization reactions are just a special type of double replacement reaction.
How does a titration work?
Measure a specific volume of your acid of unknown concentration.
Add a few drops of indicator that will change color near pH 7.
Mix a base of known concentration with the acid until the indicator changes color.
From the molarity and volume of base used, calculate the moles of acid used.
Use the moles of acid calculated and the volume of acid measured to find molarity.
Neutralization reaction shortcut formula
(# H+)MaVa=(# OH)MbVb
The # H+ and # OH are subscripts from the reaction, NOT coefficients
Percent error formula
100*|(experimental-actual)/actual|