Cancer genetics 2
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
What’re proto-oncogenes?
NON cancer causing genes
It’s a normal gene that can become an oncogene
They encode proteins involved in cell proliferation such as:
Signal transduction, Mitogenic signaling (growth factors in mitosis
What needs to occur to proto-oncogenes to become oncogenes?
They need to acquire gain of function via mutations or increased expression
What’re oncogenes?
Genes with potential to cause cancer
They function in cell proliferation, signal transduction, mitogenic signals
They upregulate gene expression (eg: transcription factors)
What’re the genetic alterations that cause proto-oncogenes and what do they result in?
Mutation: results in hyperactive protein (ex: BRAF V6OOE)
Gene amplification: results in overexpressed amounts of normal protein (ex: HER2 amplification)
Chromosome rearrangement: results in overexpressed protein, normal vs fusion (ex: BCR: ABL fusion)
What’re oncogenes in cancer?
Hypermorphic (increased) function and/or expression
UPREGULATED, mis -expressed (ectopic expression), enhanced activity/ function, etc
How do mutations act in oncogenes in cancer?
They act dominantly and are usually somatic mutations
Explain amplified (double minutes) mutations in cancer
Double minutes = not normal chromosomes, no telomeres or centromeres, highly replicated pieces of DNA
Ex: HER2/ Neu, K-Rays, c-Myc, etc
What’s the classical breast cancer oncogene?
Human epidermal growth factors receptor 2
HER2/ Neu, ERBB2
Proto-oncogene (17q21-22)
How does the classical breast cancer oncogene work?
It’s a surface bound tyrosine kinase receptor which binds to growth factors inducing dimerization and signaling (phosphorylation)
Signal transduction causes cell growth, differentiation, etc
How much do breast cancers have ERBB2 amplification?
~30% of them
Causes overactive signaling and enhanced proliferation potential
What does breast cancer causes?
Increased expression and higher disease recurrence
Causes other cancers such as ovarian, stomach, uterine, etc
What does more HER2 look like in immunohistochemistry?
More brown in later stages
What do breast cancer therapies (herceptin) aim to do?
They aim to inhibit dimerization in domain with pertuzumab and trastuzumab cuz dimerization has to occur for HER2/neu to amplify/ proliferate
Whats the normal function of tumor suppressor genes?
To inhibit/ regulate cell proliferation and work as brakes
What happens to tumor suppressor genes in cancer?
They’re down regulated/ inactivated in cancers cuz you have to lose both alleles
They have a recessive effect at cellular level because you have to lose both copies
Require loss of both functional alleles generally
How are tumor suppressor genes dominantly inherited?
Via familial cancers through loss of heterozygosity (LOH), becuase in familial cancers through, every cell in body already has 1 hit, so you js need one more hit
What’re some tumor suppressor genes?
TP53, RB1, APC
Whats TP53 (p53)?
The classical TSG (guardian of the genome)
Mutated in >50% of all cancers (has key importance in a lot of functions)
Whats retinoblastoma (RB1)?
It’s a TSG
Involved in cell cycle regulation, chromatin remodeling, apoptosis
Whats retinoblastoma (RB)
A tumor suppressor genes
Cancer of the retina
Caused by loss of heterozygosity which would make it a familial cancer
Whats knudsons 2-hit hypothesis?
States that 1 mutation is constitutional (inherited), 2nd allele is mutated somatically (2nd hit) starting the process of tumorigenesis for famililial cancers
Cancer is the result of accumulated mutations
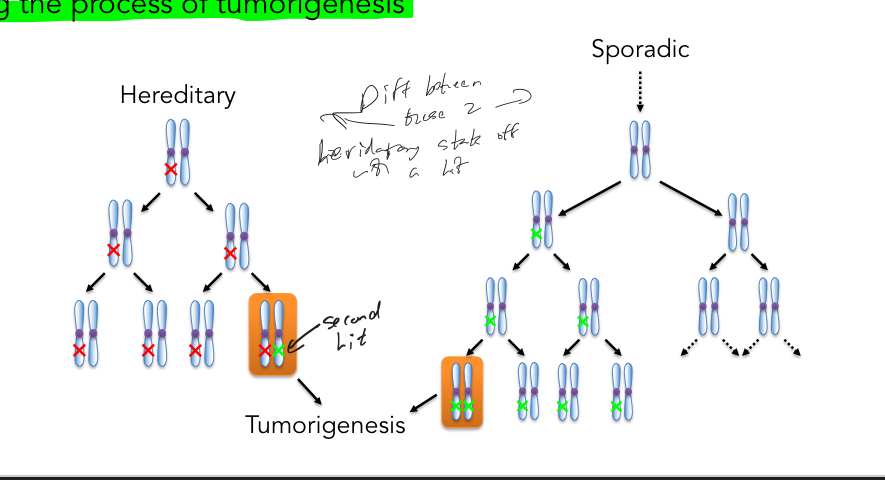
What is latency (time which cancer arises) for familiar and sporadic cancers and why?
Early for familial, later for sporadic
This is becuase of probabilities, its less likely to get the 2 hits via sporadic
What is occurrence of cancer for bi-lateral and uni-lateral for RB and why?
Bi-lateral = hereditary , Uni-lateral = hereditary or sporadic
This is becuase of probabilities, its less likely to get the 2 mutations via sporadic
How does double stranded break repair (DSB) occur?
Naturally occurring due to:
V(D)J recombination - B cells - Antibody diversity
Collapsed/ stalled replication forks
Reactive oxygen species (ROS)
Can also be caused by exogenous sources such as ionizing radiation
What’re the 2 types of DSB repair?
Non-homologous end joining (NHEJ)
Homologous recombination repair (HRR)
Explain Non homologous end joining (NHEJ)
Error prone repair
Glues ends back together which results in micro deletions and can promote translocations
Can occur through cell cycle
Predominant DSB repair pathway in mammals
Explain homologous recombination repair (HRR)
Error free repair
Copies a template to repair the damage, utilizes sister chromatids as template
Restricted to specific cell-cycle stages of G2 and S because it needs completed sister chromatids to use as template
More prominent than NHEJ during these stages
Secondary DSB repair pathway in mammals
Whats genome instability seen by?
Chromosome Instability - CIN
Whats CIN (chromosome instability) defined as?
Increased rate of chromosomes or large parts are gained/ lost
Very large genetic changes (whole chromosome)
Multiple oncogenes, tumor suppressor and DNA repair genes
Why do we care about CIN?
It’s highly prevalent in numerous cancer types (>80% of all cancers have shown CIN)
It’s an early event that drives tumorigenesis/ oncogenesis
Not all cells in tumor are same which is why theres drug resistance
Associated with highly aggressive tumors, multi drug resistance, poor patient prognosis
How is CIN assessed or measured?
DNA content analysis
Cytogenetic and karyotypic analyses
Chromosome ideogram
Explain what DNA content analysis does
Uses flow cytometry which analyzes DNA content NOT chromosome number
It fluorescently labels DNA, it only fluoresces when associated with DNA
Increase in fluorescence = increase in DNA content
What happens to peaks in flow cytometry?
Abnormal amounts of chromosomes causes broadening of peaks
Looks at DNA content NOT # of chromosomes
Whats cytogenetic and karyotypic analyses used for?
To evaluate numerical vs structural anomalies
Numerical - enumerate
Structural - label specific chromosomes and visually identify gross chromosomal rearrangements
Specifically assess chromosome numbers ad types
Requires mitotic preparation to be generated
Cannot be used for interphase cells
Counterstain and/or fluorescently labels DNA
Explain what chromosome ideograms do
Groups chromosomes in size from large to small
Shows centromeres position
Depicts the binding patterns which are unique and specific for each chromosome
Whats the DNA counterstain used for in cytogenetics?
To count chromosomes
Quick and cheap
Very easy to use for liquid cancer but impossible for solid tumors cuz of cellular mass predominantly in interphase
Whats chromosome specific labeling used for in cytogenetics?
Too enumerate AND evaluate structural defects but defect must be large enough (> 1Mb of DNA)
Can’t detect inversions
More expensive and time consuming
But can use whole chromosome paints (WCP) which is chromosome specific and Special Karyotyping (SKY) which simultaneously and differently labels all chromosomes
Whats FISH used for?
To fluorescently label probes to genomic DNA
Whats the limitation/ drawbacks of FISH?
Very limited/ gene specific evaluation (BCR: ABL)
Can be used to assess mitotic and interphase cells
In terms of targeting the aberrant genetic origins of cancer whats the approach of targeting oncogenes
Targeting oncogenes is the classical approach cuz oncogenes are associated with amplification, enhanced promoter function, ectopic expression, fusion protein, etc
Aberrant Function (hypermorphic function)
BCR:ABL
Aberrant expression (hypermorphic expression)
B-RAF
What did company Novartis do?
Used a tyrosine kinase inhibitor STI-571 which popped into active site and prevented phosphorylation from happening
What does phase 1 mean?
Designed to be small clinical trials of late stage disease people basically donating their life to see if theres any adverse affects, not cure themselves but for next gen
Explain targeting B-RAF
BRAF is an oncogene, a serine/theronine kinase involved in signal transduction
In the MAPK pathway
Mitogens Activated Protein Kinase
Pro-proliferation
Anti-apoptotic
Whats the MAPK pathway and cancer?
Constitutive pathway activation (proliferation/anti-apoptotic program)
Explain B-RAF and Melanoma
Metastatic Melanoma (skin cancer)
5-year survival = 5-22% (stage 4)
B-RAF V600E - constitutive (ectopic) activity
Caused by extracellular stimulus (Mitogens) initiates signaling cascade to induce alteration in gene expression (i.e response)
What does MEK1 mutant do in MAPK pathway?
It bypassed and started being resistant to MEK1 inhibitor
How is targeting defective tumor suppressor and DNA repair genes done?
Via synthetic lethality
Explain synthetic lethality
A RARE lethal combination of 2 independently viable mutations
Extensively studied in model organisms (yeast)
Now being expanded into cancer contexts
BRCA1 and PARP (breast and ovarian cancer) 2005
Whats the mech of synthetic lethality?
Exploit the molecular defect in tumor suppressor or DNA repair gene by downregulating/ inhibiting a 2nd gene (synthetic lethal interactor = candidate drug target)
What’re some genetic interactions of synthetic lethality?
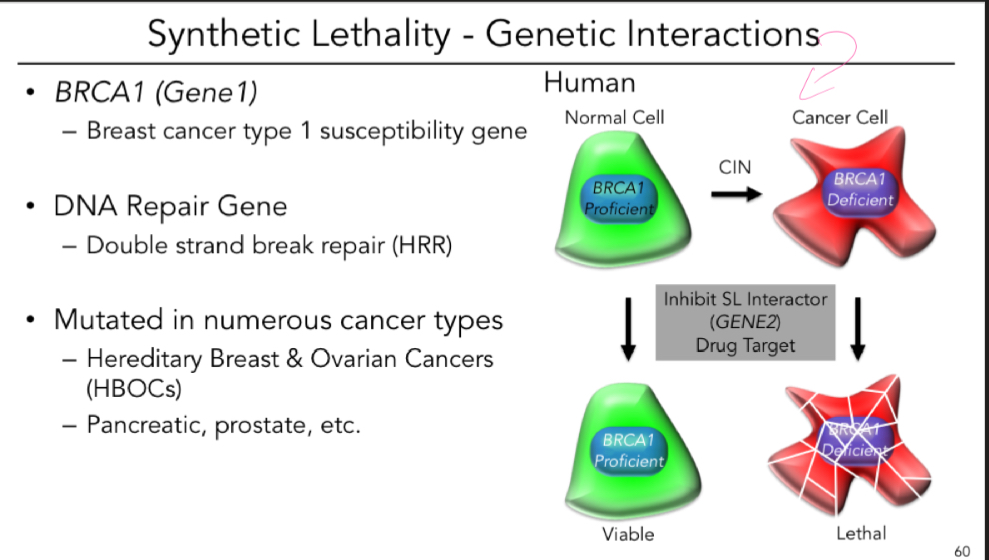
Whats the main summary of what cancer is?
