Microbiology Exam 3: Lesson 9-12
1/110
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
111 Terms
genetics.
The study of heredity and how traits are passed from one generation to the next.
What is a genome?
he complete set of genetic material in an organism
What is a gene?
A specific DNA sequence coding for a protein or RNA
What is the genetic code?
The relationship between DNA codons and amino acids
What is genomics?
The study of entire genomes
How does the bacterial genome differ from the eukaryotic genome?
Bacteria: single circular chromosome, often with plasmids, no nucleus.
Eukaryotes: multiple linear chromosomes, contained in a nucleus, with histones. some chromosomes in chloroplast and mitochondrion. (plasmid can be found in some fungi and protozoa)
When does DNA replication occur?
Right before cells divide
Each new DNA molecule contains one original (parental) strand and one newly synthesized strand.
Each new DNA molecule contains one original (parental) strand and one newly synthesized strand.
What is meant by semiconservative replication?
Each new DNA molecule has one parental strand and one newly synthesized strand
Which enzymes are key in replication?
Helicase (unwinds DNA) and DNA polymerase (synthesizes new strand)
Central Dogma
how a bacterial cell makes DNA into mRNA.
Identifies the flow of genetic material
DNA→ RNA→ protein
This involves transcription (DNA to RNA) and translation (RNA to protein).
Types of RNA
mRNA
tRNA
rRNA
What enzyme performs transcription?
RNA polymerase (separates the DNA strands apart)
How does RNA pair with DNA bases?
A–U, G–C.
Does not need any primer
Describe the process of transcription.
RNA polymerase binds to promoter and unwinds DNA.
Uses one DNA strand as template; base pairing: A–U, G–C.
No primer needed.
DNA behind polymerase rewinds.
RNA polymerase reaches terminator, mRNA is released, DNA helix reforms
What is the difference between vertical and horizontal gene transfer?
Vertical gene transfer: Transmission of genetic material from parent to offspring during reproduction.
Horizontal gene transfer: Transmission of genetic material between organisms in the same generation (not parent-to-child). Important in bacteria for genetic diversity
Describe the process of translation.
Ribosomes read mRNA in triplets (codons).
Start codon: AUG.
Stop codons: UAA, UAG, UGA.
tRNA anticodons pair with codons, bringing correct amino acids.
Amino acids joined to form polypeptide until stop codon reached.
Protein is released
How does gene expression differ in eukaryotes vs prokaryotes?
Prokaryotes: Transcription and translation occur simultaneously in cytoplasm. Genes often arranged in operons. No introns.
Eukaryotes: Transcription occurs in nucleus, translation in cytoplasm. Genes usually regulated individually. mRNA is processed
How can microbes be used in genetic engineering for medicine?
Insert the gene coding for desired protein into bacteria.
Bacteria transcribe and translate the gene.
Protein can be harvested (e.g., insulin, vaccines)
Bacterial Regulation Through Operons
Regulates when specific genes are expressed (transcribed and translated)
Allows for quick adaptation to environmental conditions without wasting energy.
Use operons
Promoter
RNA polymerase binding site.
Operator
Controls RNA polymerase access to genes.
Operon
Group of genes with related functions. have the “on/off switch”
Inducer
Molecule that activates gene expression.
Repressor
Protein that blocks gene expression.
Plasmid
Extra-chromosomal DNA in bacteria.
Structural genes:
Code for enzymes in a metabolic pathway.
Regulatory gene
Produces a repressor protein
What are the parts of a bacterial operon and their functions?
Promoter: Binding site for RNA polymerase.
Operator: Controls RNA polymerase access to genes.
Structural genes: Encode enzymes in metabolic pathway.
Regulatory gene: Produces a repressor protein.
Operons allow rapid adaptation to environmental changes w/o wasting energy
Inducible Operon
An operon that is normally OFF but can be turned ON when needed.
Why? Because the genes make enzymes for something that isn’t always around.
describe lac operon (inducible operon)
Codes for enzymes that digest lactose.
OFF by default.
When lactose is present, it acts as an inducer, inactivating repressor → operon ON → RNA polymerase can enter → transcription & translation of enzymes that digest lactose
Key point: The lac operon is inducible (off by default, turned on when lactose is present).
Repressible Operon
Definition: An operon that is normally ON but can be shut OFF if enough product is already present.
Why? Because the cell usually always needs the product, but doesn’t want to overproduce it.
Describe the trp operon (repressible operon)
Codes for enzymes that synthesize tryptophan.
ON by default.
When tryptophan is abundant, it acts as a co-repressor, activating repressor → operon OFF → enzymes not produced, tryptophan synthesis stops
Key point: The trp operon is repressible (on by default, but can be shut off when enough product is around).
How do bacteria respond to environmental changes like excess tryptophan?
Feedback inhibition: Regulates enzyme activity.
Gene regulation: Stops production of enzymes (e.g., turning off trp operon)
What factors contribute to genetic diversity in prokaryotes?
Rapid reproduction (binary fission).
Mutations.
Horizontal gene transfer (HGT).
Allows sharing of virulence and antibiotic resistance genes
three types of horizontal gene transfer in bacteria
transformation, conjugation and transduction
What is bacterial transformation?
Uptake of naked DNA fragments from environment.
Does not require cell-to-cell contact.
Contributes to antibiotic resistance.
Example: Streptococcus pneumoniae acquiring resistance from dead cells
What is bacterial transduction?
Transfer of bacterial DNA between cells via a bacteriophage (virus).
No direct contact required.
types: generalized transduction and specialized transduction
Generalized transduction:
Bacteriophages accidentally package bacterial DNA instead of viral DNA → can transfer any gene.
Specialized transduction:
Prophage integrates into bacterial genome, and upon activation, carries specific host genes to new cells
What is bacterial conjugation?
Direct transfer of DNA between two bacterial cells through a sex pilus.
Requires physical contact.
Transfers plasmids and/or chromosomal DNA.
Major contributor to antibiotic resistance
Compare F+ × F– vs Hfr × F– conjugation.
F+ × F–: Donor cell (F+) transfers plasmid DNA to recipient (F–), converting it into F+. Only plasmid DNA is transferred.
Hfr × F–: F plasmid integrates into donor chromosome. During conjugation, part of the chromosomal DNA is transferred to F– cell (recipient). F– usually remains F– because not all plasmid DNA transfers
How can bacteria be engineered to produce human insulin?
Insert the insulin gene into a plasmid, introduce it into bacteria, then allow transcription & translation to produce insulin protein
Define sterilization.
Complete elimination of all vegetative cells, endospores, and viruses from inanimate objects (e.g., surgical instruments, needles)
Define disinfection.
Removes or destroys pathogens from inanimate objects though application of heat or antimicrobial chemicals (e.g., lab benches, clinical surfaces)
Define antisepsis.
Disinfection of living tissue (e.g., pre-surgery skin prep, wound cleaning)
Define degerming.
Mechanical removal of microbes from a limited area (e.g., handwashing, alcohol swabs)
Define sanitization.
Lowering microbial counts to safe public health levels on inanimate objects (e.g., restaurant dishes, restrooms)
Define asepsis.
Absence of significant contamination; applied to living or inanimate objects (e.g., sterile surgical procedures)
Define biocide/germicide.
General term for antimicrobial agents that kill microbes (on living and inanimate objects)
Define bacteriostasis.
Inhibition of bacterial growth without killing (e.g., refrigeration, some antiseptics)
What is sepsis vs asepsis?
Sepsis: Microbial contamination.
Asepsis: Absence of significant contamination (goal in surgery).
Define commercial sterilization.
Killing Clostridium botulinum endospores in food processing
What factors influence effectiveness of microbial control?
Number of microbes (high # of microbes = longer exposure/stronger concentrations needed).
Environment: organic matter, temperature, biofilms.
Organic matter (blood, pus, food) can bind to disinfectants and reduce effectiveness
Biofilms act as protective barriers.
Time of exposure.
Microbial characteristics (spores, mycobacteria, cysts, etc.)
What are the main cellular targets of microbial control agents?
Cell membrane
Proteins
Cell wall
Cellular Synthesis (Nucleic acids Damage DNA/RNA (e.g., radiation, some chemicals).
microbial control agents affect on cell membrane
Agents physically bind to lipid layer of the cell membrane, opening up the cell membrane and allowing injurious chemicals to enter the cell and important ions to exit the cell.
Alter permeability (e.g., alcohol, detergents).
microbial control agents affect on cell wall
Blocking synthesis or digesting cell wall
microbial control agents affect on cellular synthesis
Agents can interrupt the synthesis of proteins via the ribosomes, inhibiting proteins needed for growth and metabolism and preventing multiplication.
Agents can change genetic codes (mutation).
microbial control agents affect on protiens
Denaturation or blocking active sites (e.g., heat, formaldehyde).
Compare moist heat vs dry heat.
Moist heat (better at denaturing proteins):
Boiling: Kills many microbes but not reliable against spores.
Autoclaving: Steam under pressure (121°C, 15 min) = true sterilization.
Pasteurization: Reduces pathogens and spoilage organisms but doesn’t sterilize
Denatures proteins, sterilizes surgical instruments and media.
Dry heat: Kills by oxidation (dry heat, flaming, incineration, hot-air ovens). Requires higher temp & longer exposure bc it lack the protein denaturing steam of moist heat
How do filtration, low temperatures, high pressure, desiccation, and osmotic pressure suppress microbes?
Filtration: removes microbes from heat-sensitive liquids/air.
Low temps: slow metabolism (refrigeration, deep-freezing, lyophilization).
High pressure: denatures proteins, used in food preservation.
Desiccation: removes water, halts metabolism.
Osmotic pressure: salt/sugar → plasmolysis, dehydration, death.
What is the difference between chemicals for antisepsis vs disinfection?
Antisepsis: chemicals safe for living tissue (iodine, alcohol, hydrogen peroxide).
Disinfection: chemicals for inanimate objects (bleach, phenols, Lysol).
What is the action of heat on microbes?
Destroys proteins, nucleic acids, removes water.
What is the disk-diffusion method?
A test where chemical-soaked disks are placed on microbial lawns; measure inhibition zones to evaluate effectiveness.
Define spectrum of activity.
The range of pathogens on which a drug is effective.
What is a broad-spectrum antibiotic?
Works against many types of pathogens (Gram-positive and Gram-negative).
What is a narrow-spectrum antibiotic?
Works against a limited range of pathogens.
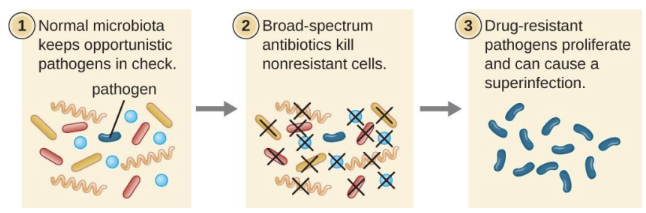
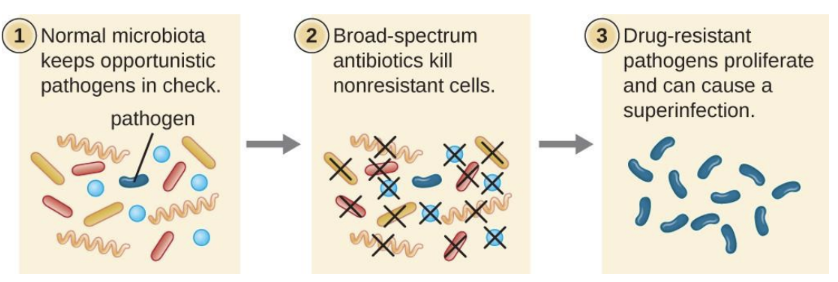
What is a superinfection?
A secondary infection caused when broad-spectrum antibiotics eliminate normal protective microbiota, allowing resistant or opportunistic microbes to overgrow (e.g., C. difficile colitis, Candida yeast infection)
chemotherapy
the use of drugs to treat a disease
selective toxicity
a drug should harm the pathogen but not the host
The toxic dose = harm to the host
The therapeutic dose = eliminates pathogens in the host
superinfections examples
Urinary tract infection caused by E. coli treated with antibiotics
lactobacilli in the female vagina are killed by the broad-spectrum cephalosporin used to treat the UTI
overgrowth of Candida albicans occurs, causing a vaginal yeast infection or oral thrush
Antibiotic-associated colitis
oral therapy with broad-spectrum antibiotics kills off normal biota of the colon.
overgrowth of Clostridium difficile invades the intestinal lining and releases toxins that cause diarrhea, fever, and abdominal pain
More than half of antibiotics are produced by what organisms?
Species of Streptomyces (a group of soil bacteria).
What are the ideal properties of antimicrobial drugs?
Selective toxicity (kills pathogen, not host).
Microbicidal (kills microbes, not just inhibits) rather than microbistatic.
Remains potent long enough.
Active in tissues and body fluids.
Easily delivered to infection site.
Doesn’t trigger resistance easily
reasonably priced
does not disrupt the host health by causing allergies or predisposing the host to other infections
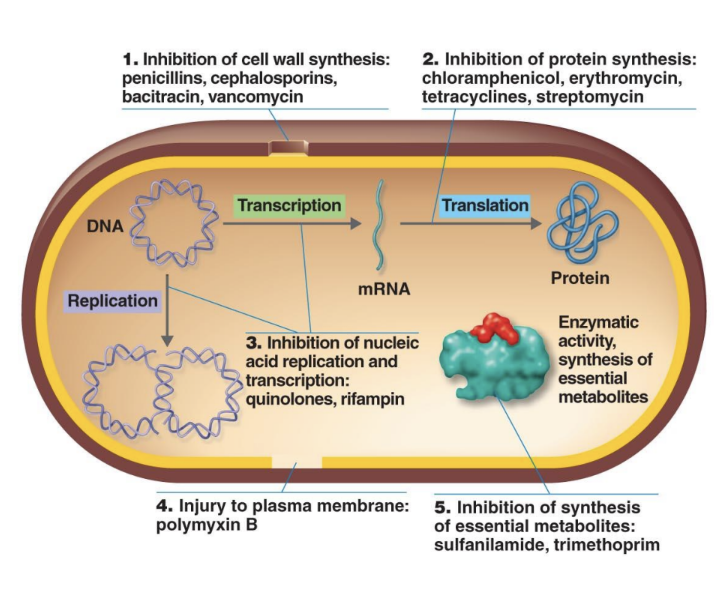
What are the five modes of antibacterial drug action?
Inhibit cell wall synthesis (penicillins, cephalosporins).
Inhibit protein synthesis (tetracyclines, macrolides).
Inhibit nucleic acid synthesis (rifampin, quinolones).
Disrupt plasma membranes (polymyxins).
Inhibit metabolic pathways (sulfa drugs).
How do antifungal drugs work?
Damage membranes and cause content leakage.
Nystatin: Treats Candida albicans.
Amphotericin B: Treats systemic fungal infections.
How do antiviral drugs work?
Block viral entry, inhibit nucleic acid synthesis, or prevent assembly/release of viral particles.
Why is it hard to target viruses without damaging host cells?
Viruses use host cell machinery for replication, so drugs risk harming host cells as well
goal of antiprotozoal drugs
is to eradicate the parasite
include aminoquinolines, nitroimidazoles, and fexinidazole
How do aminoquinolines (quinine) work?
Accumulate in parasitized RBCs, block parasite’s ability to digest hemoglobin (used for malaria).
How do nitroimidazoles work?
Interfere with DNA synthesis. Used against amoebiasis, giardiasis, trichomoniasis.
What is fexinidazole?
: Recently approved drug for African trypanosomiasis (sleeping sickness); safer than older arsenic-based drugs
How do antihelminthic drugs act?
Praziquantel: Alters membrane permeability in cestodes and trematodes, causing paralysis in the parasite.
Mebendazole: Inhibits nutrient uptake.
Ivermectin: Causes paralysis in roundworms
Define MIC
MIC (Minimal Inhibitory Concentration): Lowest concentration of drug that inhibits visible growth.
Define MBC.
MBC (Minimal Bactericidal Concentration): Lowest concentration that kills bacteria (no colonies grow on subculture).
What is the disk-diffusion method (Kirby-Bauer)?
Disks with antibiotics placed on agar inoculated with bacteria; measure zones of inhibition to test susceptibility
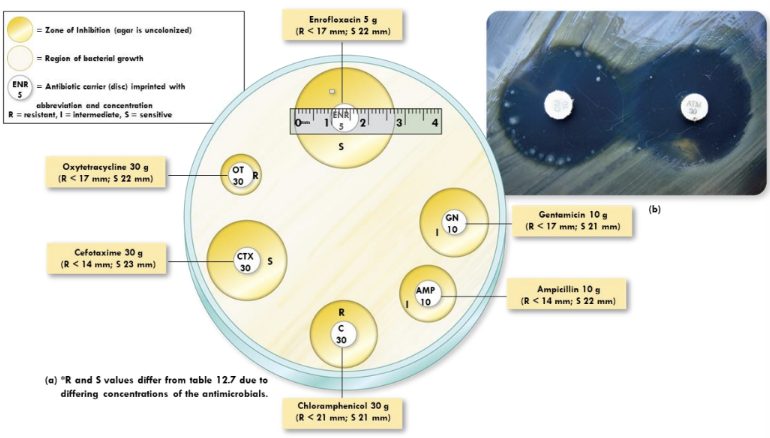
What is the tube dilution test?
more sensitive and quantitative than the Kirby Bauer test
Serial dilutions of drug in broth to find MIC. Subculturing can reveal MBC
Why do bacteria become resistant to antibiotics?
Genetic mutations.
Acquisition of resistance genes via horizontal gene transfer (plasmids, transposons).
What human behaviors encourage antibiotic resistance?
Overuse of antibiotics.
Antibiotics being available over the counter in developing countries encourages this
Not finishing prescriptions.
Patient non-compliance (not completing full antibiotic courses) leaves surviving bacteria—often the most resistant ones—to multiply and spread
Antibiotics in livestock feed
can be transmitted to humans through meat consumption
What are the two primary tests for microbial susceptibility to chemotherapeutic agents?
the disk-diffusion (Kirby-Bauer) test and the broth dilution test
Why aren’t broad-spectrum antibiotics always ideal?
They disrupt normal microbiota, causing superinfections and microbiome imbalance.
Superinfection
A secondary infection that occurs when broad-spectrum antibiotics eliminate normal protective microbiota. This allows resistant or opportunistic pathogens to overgrow and cause disease.
For example, killing lactobacilli in the vagina with a broad-spectrum cephalosporin used for a UTI can lead to an overgrowth of Candida albicans, causing a vaginal yeast infection
What mechanisms do bacteria use for resistance?
Altered metabolic pathways.
Enzymatic inactivation of drugs.
Efflux pumps expel drugs.
Target site mutations (e.g., ribosomes).
Fomite
inanimate objects that can carry and spread disease and infectious agents.
Contamination with an infectious agent, like bacteria or a virus.
Acts as a vehicle for disease transmission.
Pathology
The study of disease
Etiology
The study of the cause of a disease
Pathogenesis
The development of disease
Infection
The successful colonization of the body by pathogens
Disease
Any condition in which the normal structure or functions of the body are damaged or impaired
Transient microbiota
Microbes that may be present for days, weeks, or months
Normal microbiota
Microbes that permanently colonize the host
Symbiosis
The relationship between normal microbiota and the host