Lecture #5 | Chemical Causes of Cancer III Phase II, Phytochemicals, Aflatoxin B1, Dioxin and AhR Receptor-mediated toxicity
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
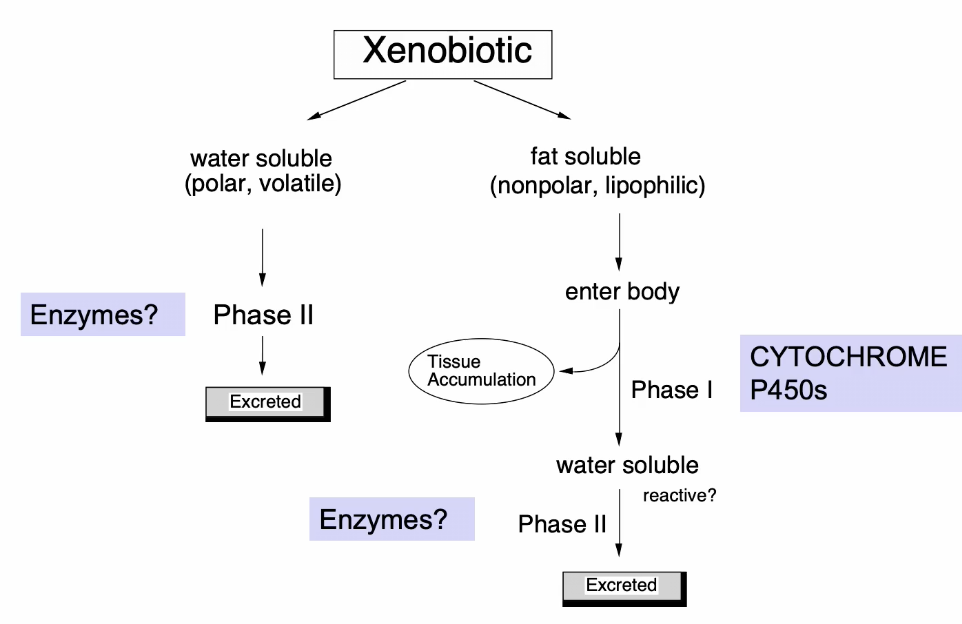
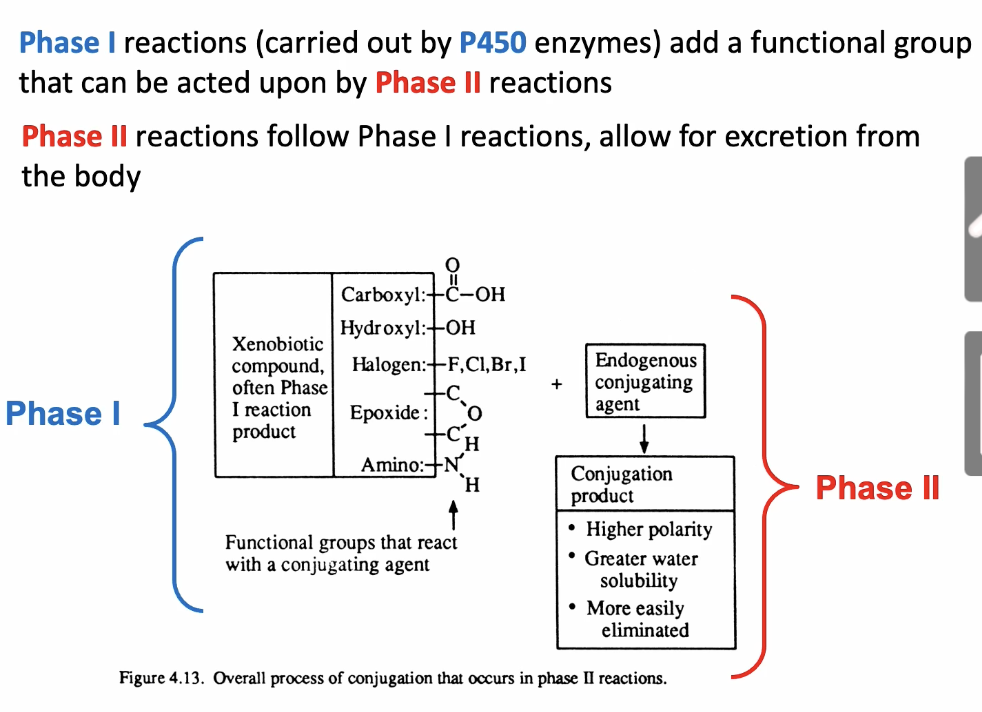
Phase II reactions
Conjugated reactions (chemically linked) to create a conjugate
Conjugates are:
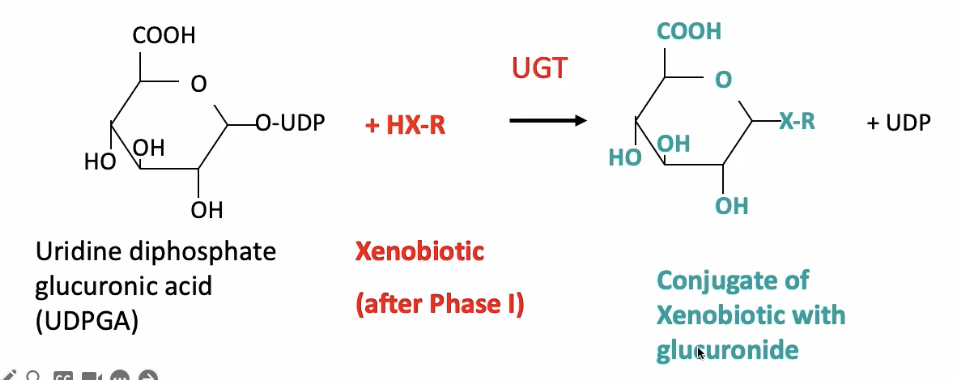
Glucuronides: most common, sugar + nucleotide
Glutathione: peptide (Glu-Cys-Gly)
Sulfhydryl-SH
Conjugation with glucuronide

Sugar + Nucleotide interacts with a xenobiotic that has already went through Phase 1 (has a polar group)
A transferase will mediate the xenobiotic with the glucuronide
Molecule is too big and will be targeted for removal
Conjugate with gluthathione
Binds to electrophiles due to SH group in system to help remove radicals
Beneficials
Detox
Antioxidant
Protects against environmental toxin
Harm
1. Cancer Risk due to stoppage of apoptosis
May block chemotherapy drugs
Compounds that induce Phase 1 and Phase 11
Constitutive and inducible forms of both Phase 1 an phase 2 enzymes
example of both is phytochemiclas
Monofunctional: induce just phase 2
Bifunctional: induces Phase 1 and 2
Phytochemicals
Chemicals in plants
help prevent cancer
impact has 1/11
Flavonoids: major class
Water soluble pigments
Can decrease risk of variety of cancers
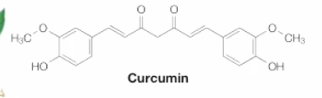
Curcurmin
Phyochemical from turmeric
Capsaicin
Phyochemical from chili peppers
Epigallocatechin-3-gallate
phytochemical from green tea
flavonoids
Genistein
Phytochemical from soybeans
flavoidoids
Lycopene
Phytochemical found in tomatoes
Resveratrol
Phytochemical found in grapes
inhibits all 3 stages of cancer
Indole-3-carbinol
phytochemical found in cabbage
Sulphoraphane
Phytochemical found in broccoli
Flavones
mechanisms for chemopreventative properties of phytochemicals
induced detoxification enzymes (both phase 1 and 2)
blocks the formation of carcinogens by acting as anti-oxidants
act as a tumor suppressing agents
antagonize the effects of estrogens → growth
stimulate DNA repair
How do phytochemicals act as anti-carcinogens
Increasing carcinogens detoxification
Suppressing the chemical activation of procarcinogen to ultimate carcinogen
Suppressing the promotion and progression of carcinogen
Alfatoxin B1
Produces by a mold that lives on peanuts and corn
mainly found in Africa and Asia
Originally identifies in England
Metabolically activated to a carcinogen by P450 enzyme
100x more mutagenic and carcinogenic than B[a]P
Metabolic activation of aflatoxin B1
Alfatoxin is ingested and converted into aflatoxin B1-8,9 oxide by P450 through a phase 1 reaction
If it is acted upon by epoxide hydrase (water added), it is detoxified
If it comes into contact with glutathione S-transferase it can undergo a phase 2 via GST (also not harmful)
However, aflatoxin B1-8,9 oxide by itself is very unstable and has a tendency to form a DNA adduct
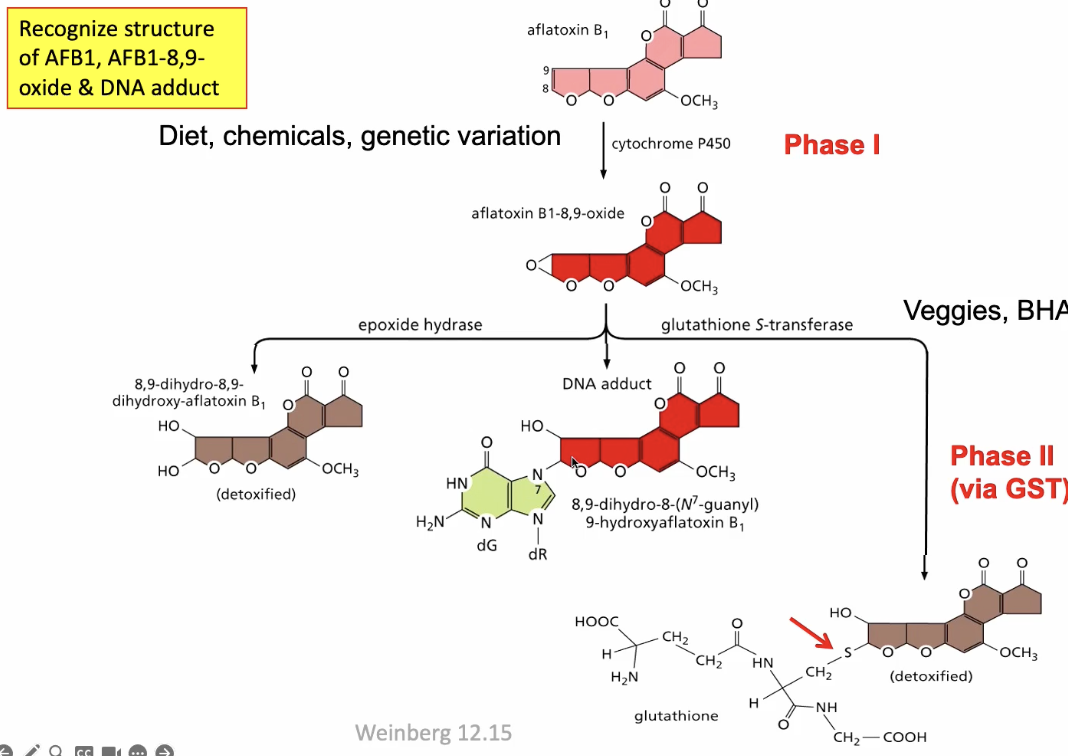
once adduct forms, it can lead to p53 which is a tumor suppressor
HBV (hepatitis b virus) infection
Acts as a tumor promoter, inducing a cell proliferation in the liver
increased proliferation of cells containing AFB1 adducts in DNA means less time for repair
More mutations
Dioxin
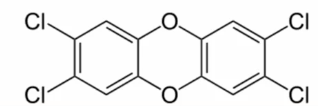
complete carcinogen
linear dose response
Low doses do matter, do accumulate
Environmental exposure of Dioxins
Never made commercially but byproducts of
Plastics, bleach, antiseptics
Incomplete combustion
chlorinated wastes
wood burning
Major increase after 1940s
Bioaccumulation of dioxins
Concentrate in the food chains and make its way to humans
from meat and dairy products
Some dioxins are present in every person on the planet
average body burn is 5-50pg/gr of body fat
Good news about dioxins
Annual emission of dioxins decreased 90% from 1987 to 2000 due to EPA regulations
decrease in intake of dioxins
Epidemiology of dioxins
Classified as a known human carcinogen in 1997
1976 explosion at chemical plats in Italy
released 3 kg of TCDD (2x annual emissions in the US in 2000)
increase in liver cancer and myelomas but decrease in breast and endometrial cancer
Fate of xenobiotics in the body