Drug Metabolism: Phase 1
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
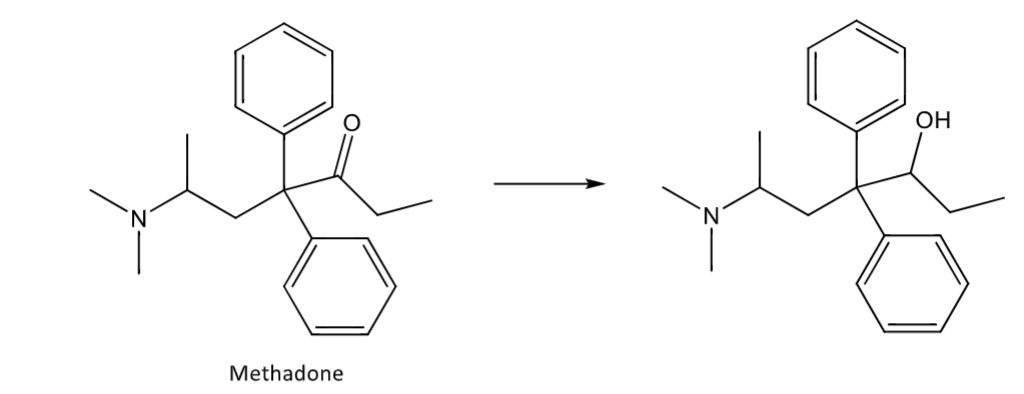
Phase 1 reactions
in vivo chemical modifications that convert the drug to a more polar moiety
Most important enzymes involved in Phase 1 metabolism
Cytochrome P450 enzymes
Flavin monooxygenases (FMOs)
Alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH)
esterases, amidases
Cytochrome P450 enzyme
superfamily with the same iron-protoporphyrin IX prosthetic group (heme cofactor) at the catalytic center
transfers an oxygen from the heme cofactor to the drug
Flavin Monooxygenases (FMOs)
involves direct oxidation of nitrogen and sulfur atoms
non-heme, instead use flavin adenine dinucleotide (FAD) prosthetic group
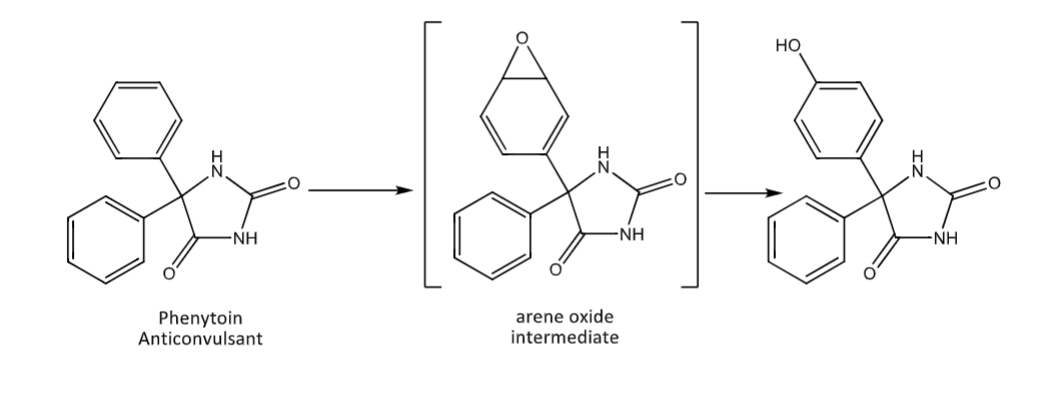
Aromatic hydroxylation
CYP enzyme mediated
usually at the para position
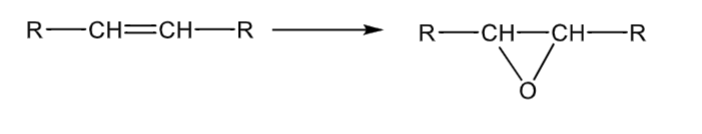
Alkene epoxidation
CYP3A4 mediated epoxide formation
can do a phase 2 reaction - epoxide hydrolase forms a trans diol, which is usually inactive
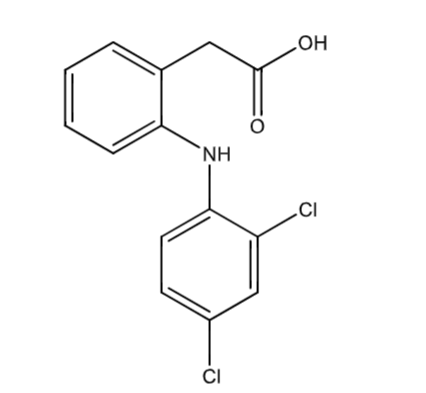
Fenclofenac
not marketed due to severe liver toxicity
modifications prevent para-hydroxylation
t ½ = 20h
Diclofenac
undergoes para-hydroxylation
t ½ = 2h
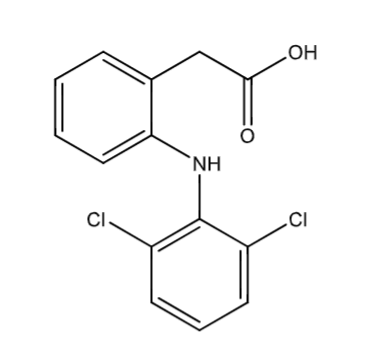
benzylic hydroxylation: sites of biotransformation
carbon directly adjacent to the benzylic ring
if the carbon already has a heteroatom (O, N, etc) then oxidation generally does not occur
if there are multiple possible sites, oxidation occurs at the least sterically hindered position
alkyl hydroxylation
occurs generally at omega or omega-1
adds an OH group
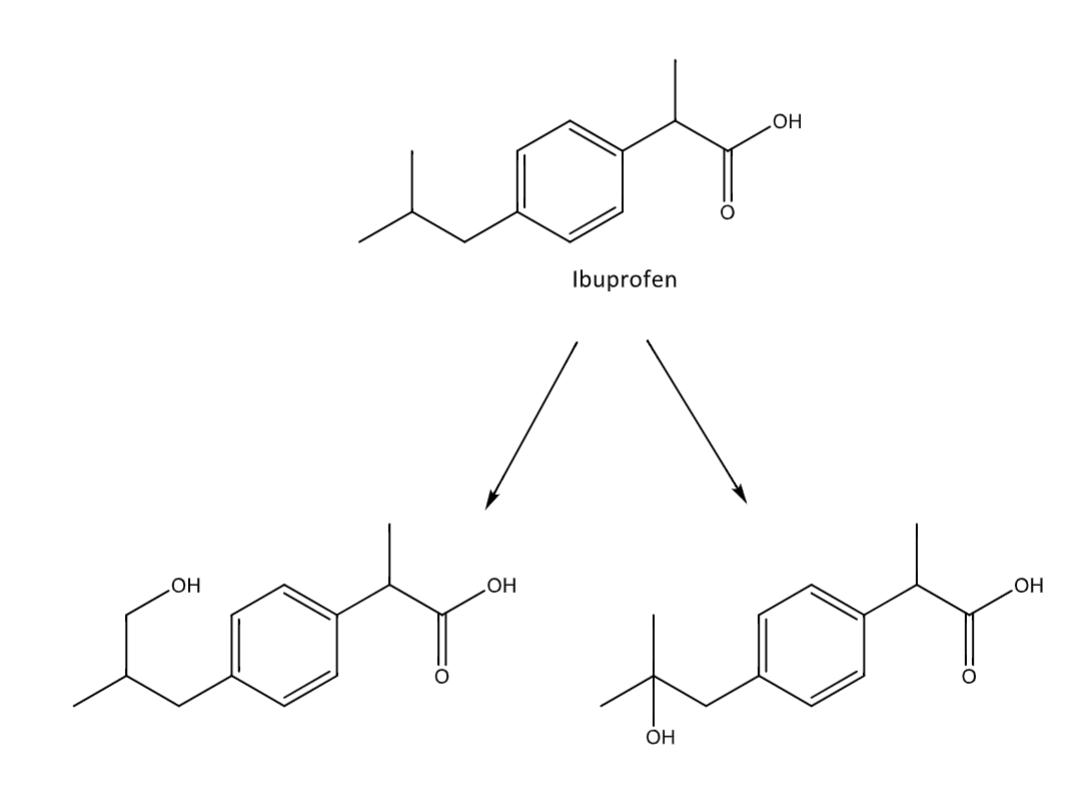
Allylic hydroxylation
occurs a carbon over from an allyl group
adds an OH group
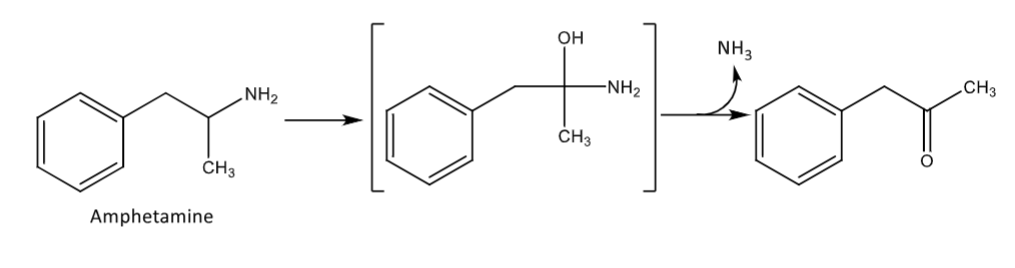
oxidative deamination
only occurs in primary alkyl amines
need at least one H on the carbon alpha to the amine
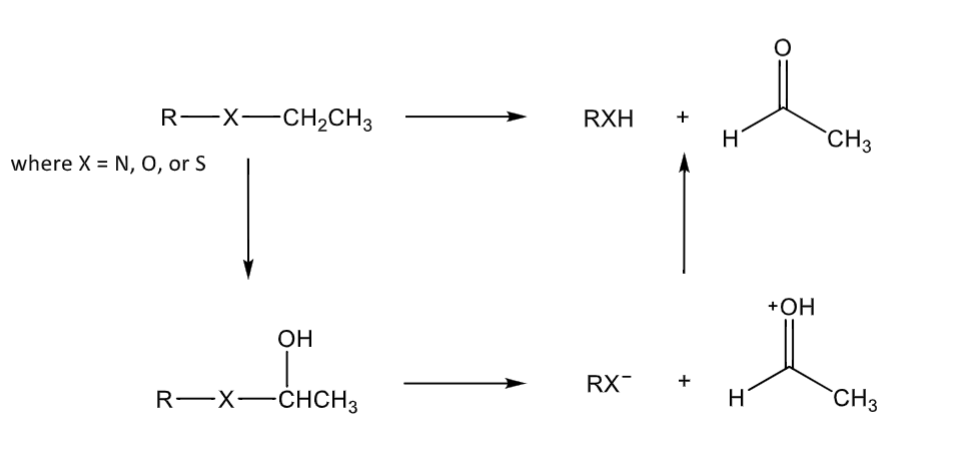
N, O, S dealkylation
hydroxylation followed by fragmentation
typical N-substituents removed are typically methyl, ethyl, n-propyl, isopropyl, n-butyl, allyl, and benzyl
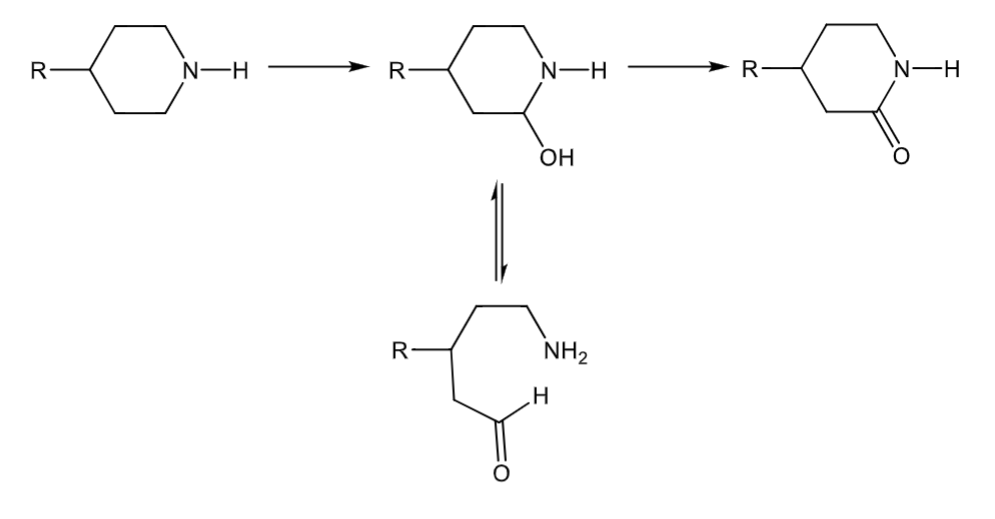
amine dealkylation: ring systems
forms a carbinolamine that can break the ring
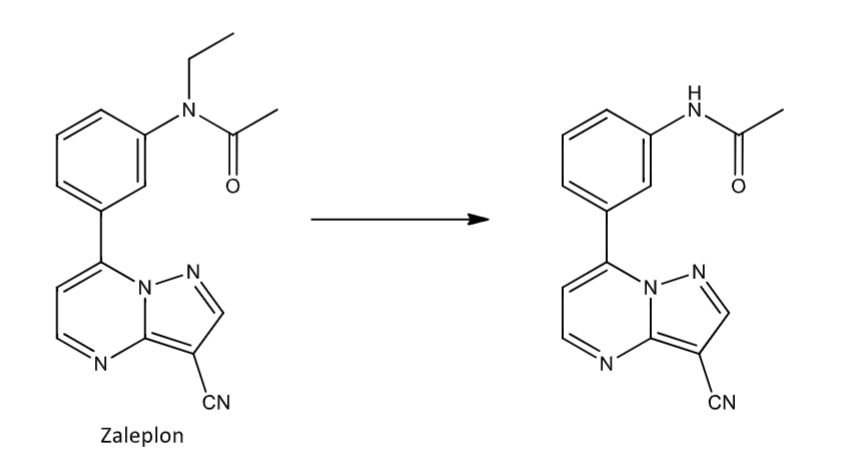
N-dealkylation of amides
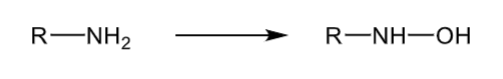
N-oxidation: primary amine
forms a hydroxylamine
can further oxidate to a nitroso group and then form an oxime
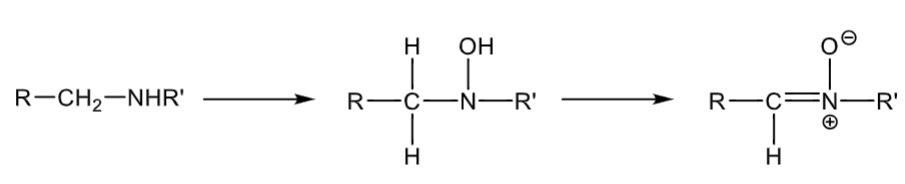
N-oxidation: secondary amines
forms a hydroxylamine
can further oxidate to form a nitrone
N-oxidation: tertiary amines
forms an N-oxide
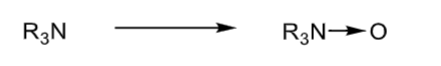
N-oxidation: notes
can also occur on:
pyridines
amides, particularly those of arylamines
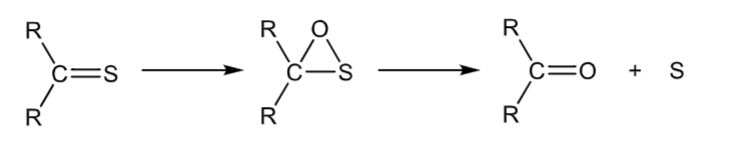
Desulfurization
thiocarbonyl to a carbonyl
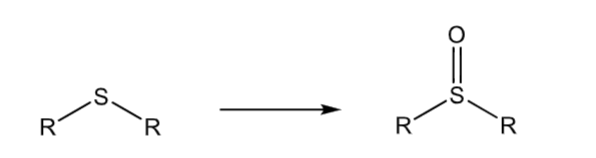
Sulfoxidation: thioethers
can further oxidize to a sulfone
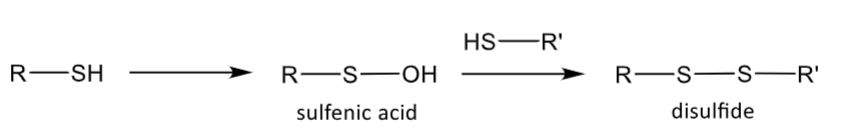
Sulfoxidation: thiol compounds
forms a sulfenic acid
this can react with another thiol and form a disulfide bridge
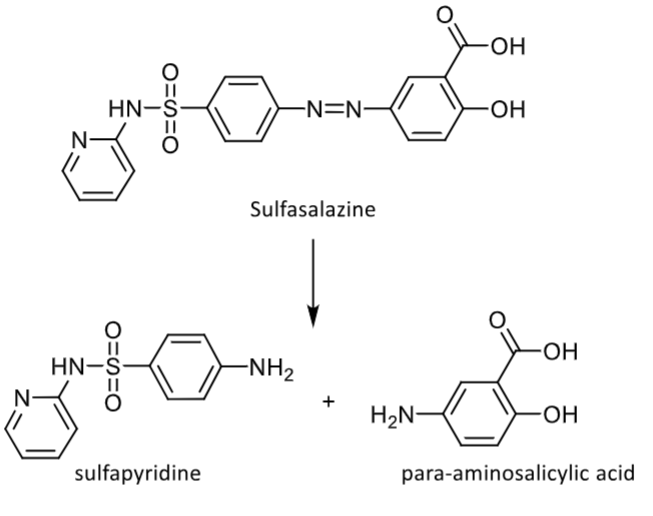
Azo reduction
cleaved by azo reductases that are produced by intestinal bacteria
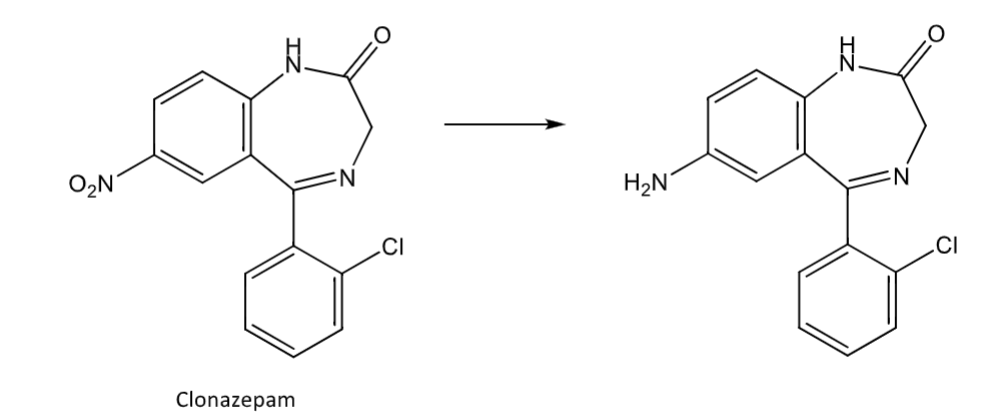
Reduction: arylnitro group
Reduction: carbonyls (Aldo-keto reductases)
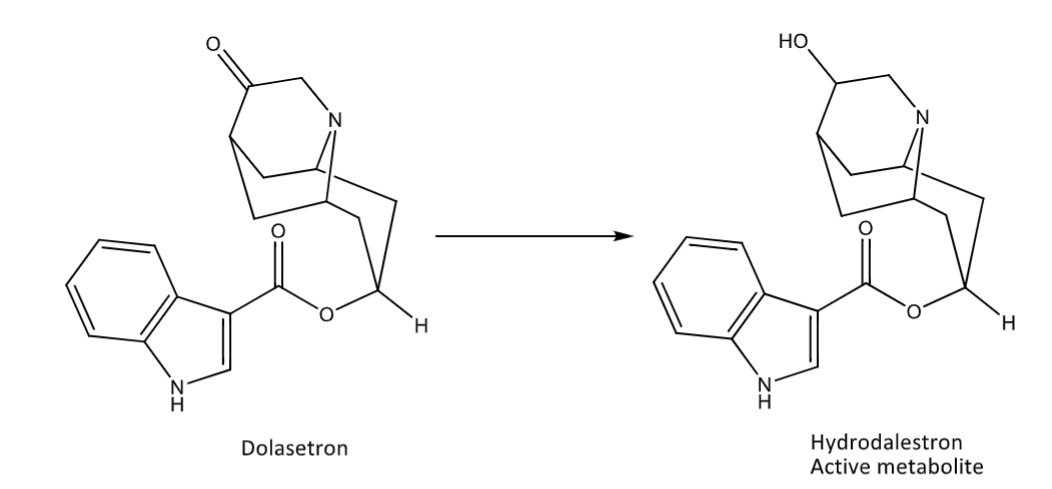
Reduction: carbonyls (carbonyl reductases)
Reduction: carbonyls (alcohol dehydrogenase)
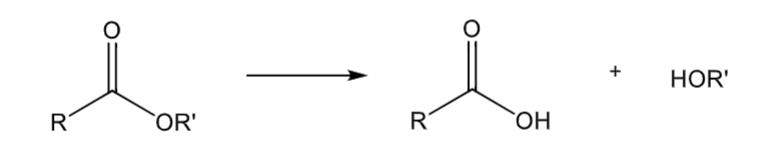
Ester hydrolysis
mostly in plasma, some in liver
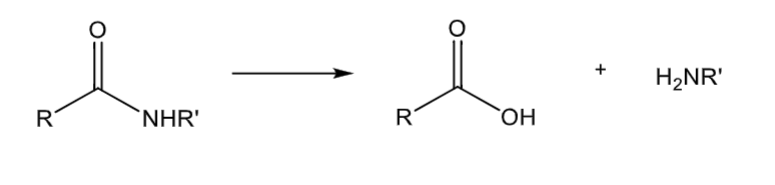
Amide hydrolysis
peptidase mediated
MAO subtypes
A - placenta, liver, gut
B - brain, liver, platelets
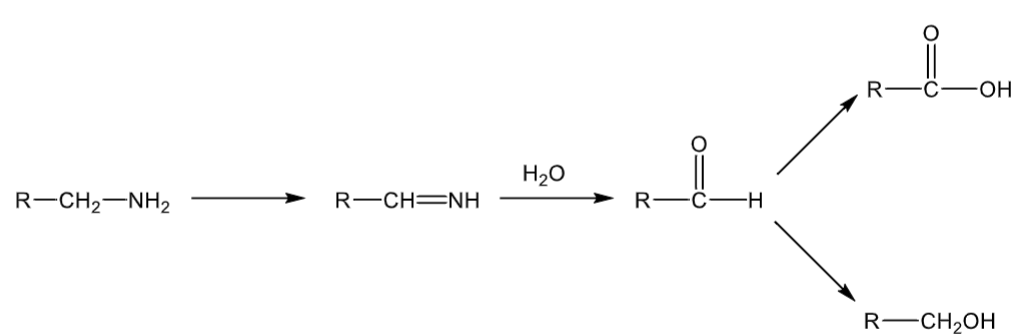
MAO reactions
Amine can be:
primary (2 Hs) or have groups no larger than a methyl
forms an imine intermediate
carbon alpha to the nitrogen cannot be CH3
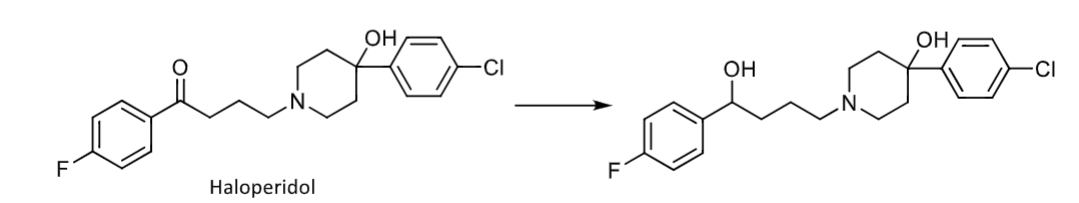
alcohol and aldehyde oxidations
mediated by dehydrogenase enzymes
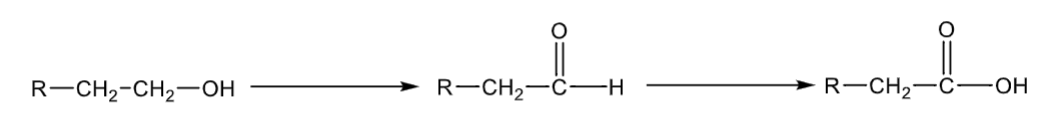
Disulfuram
“Antabuse”
used in maintenance of alcohol dependence
inhibits ALDH, increasing acetaldehyde which causes vomiting
Fomepizole
“Antizole”
inhibits ADH, preventing initial biotransformation of compounds, preventing eventual formation of toxic compound in vivo
Tx methanol and ethylene glycol poisoning
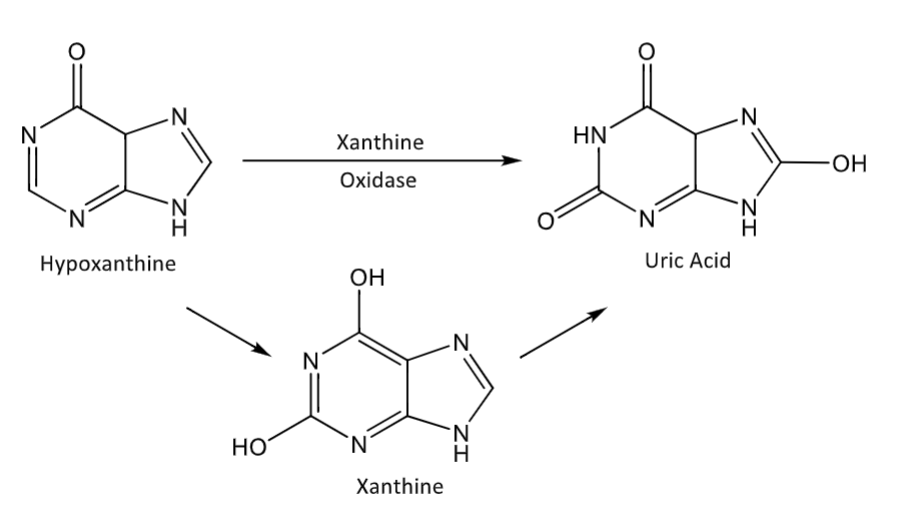
Purine oxidations