AP Chemistry 1-212
1/329
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
330 Terms
Strong acids
HCl, HBr, HI, HClO₄, HNO₃, H₂SO₄
H₂ bond energy & bond type
Single covalent bond, ~400+ kJ/mol
F₂, Cl₂, Br₂, I₂ bond energies & bond types
Single covalent bonds; bond energies range from ~150-250 kJ/mol
O₂ bond energy & bond type
Double covalent bond, ~500 kJ/mol
N₂ bond energy & bond type
Triple covalent bond, ~900 kJ/mol
Strong bases in AP Chemistry
All soluble metal hydroxides (e.g., NaOH, KOH, Ba(OH)₂)
Celsius to Kelvin conversion
K = 273 + °C
Percent error formula
%Error = [(Experimental - Correct) ÷ Correct] × 100
Importance of sign in percent error
It shows whether the measurement is above or below the actual value.
How many significant digits should you use on the AP Exam?
Three significant digits (3), for 95% of all questions. If it is a simple calculation, be careful and follow the sig fig rules.
Situations requiring attention to significant figures
When performing lab measurements using addition/subtraction or simple single operations like molar mass calculations
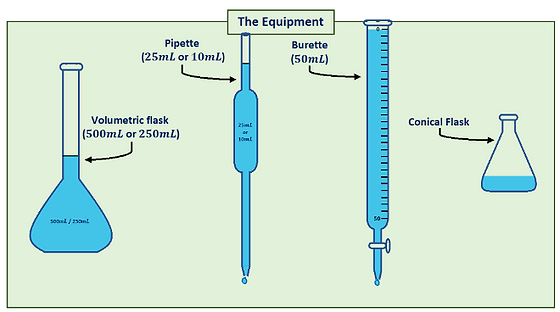
Most accurate volume-measuring devices (3 of them)
Volumetric flask (fixed volume), burette (variable volume), pipette (variable volume)
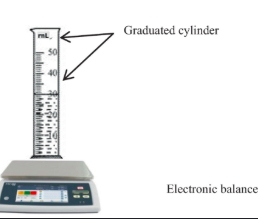
Relatively accurate lab devices (2 of them)
Graduated cylinder, electronic balance
Devices not considered accurate for volume measurements (2 of them)
Beaker, Erlenmeyer flask
Molar mass of H₂
2.0 g/mol
Molar mass of N₂
28 g/mol
Molar mass of O₂
32 g/mol
Molar mass of H₂O
18 g/mol
Molar mass of CO₂
44 g/mol
Molar mass of NaOH
40.0 g/mol
Atomic number of an element: location and meaning
The number above the element symbol on the AP periodic table; number of protons
Mass number
Mass number = number of protons + number of neutrons of an atom of a specific isotope of an element
always whole number
not shown on AP periodic table
Relationship of mass number to atomic mass
Mass number is the closest whole number to the element's atomic mass
(average) atomic mass
The weighted average of all the naturally occurring isotopes of an element; in amu or g/mol; listed on periodic table for each element.
Implication of average atomic mass close to a whole number
It means the element likely has one dominant isotope
Mass spectrometer
A tool that sorts isotopes of elements by mass and shows their relative abundance
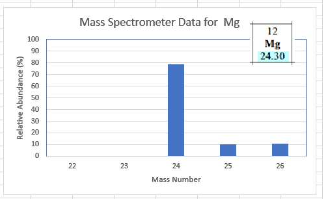
Using mass spectrometry data in AP Chemistry
Use the relative abundances and mass numbers to calculate average atomic mass; compare with periodic table value
Key differences between isotopes of the same element
They differ in the number of neutrons and their atomic mass
Coulombic forces definition
The attractions between opposite charges and repulsions of similar charges
How does sign and magnitude of charge affect Coulombic forces?
Like charges → repel; Unlike charges → attract; Greater magnitudes → stronger attraction/repulsion.
How does distance affect Coulombic forces?
Greater distance → weaker forces; Smaller distance → stronger forces.
Avogadro's number
6.02 × 10²³ particles/mol.
Limiting reactant
The reactant that determines the extent of the reaction and product amounts, found by setting up an ICE Chart and dividing mole amounts by stoichiometric coefficients.
Empirical Formula: definition
The simplest whole-number ratio of atoms in a compound.
Finding empirical formula steps
1. Find the number of mol of each element.
2. Divide all mol values by the smallest mol amount.
3. If necessary, multiply the resulting ratio to eliminate fractions.
How does the molar mass compare to the empirical molar mass?
The molar mass of a compound will be a whole number multiple of the empirical molar mass.
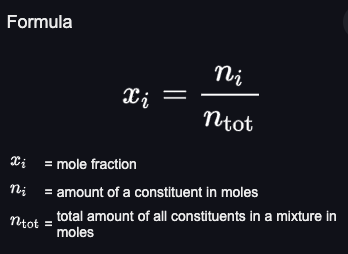
Mole fraction formula
mol amount of X / total mols
Soluble cations / anions
NO₃⁻ , Na⁺, K⁺, and NH₄⁺ form only soluble compounds and are spectator ions (not included in net ionic equations).
What unit does millimol solute / volume in mL give you?
Gives you molar concentration in mol/L (M).
Molecular equation for NaCl and AgNO₃ reaction
NaCl(aq) + AgNO₃(aq) → NaNO₃(aq) + AgCl(s).
Complete ionic equation for NaCl and AgNO₃ reaction
Na⁺ + Cl⁻ + Ag⁺ + NO₃⁻ → Na⁺ + NO₃⁻ + AgCl(s).
Net ionic equation for NaCl and AgNO₃ reaction
Ag⁺ + Cl⁻ → AgCl(s).
Weak acids and bases in net ionic equations
They are shown as molecules, not ions (undissociated, while the strong acids/bases fully dissociate).
Net ionic equation for strong acid-strong base reaction
H⁺ + OH⁻ → H₂O(l).
Convert torr or mmHg to atm
torr ÷ 760 = atm.
Metric unit for pressure
The pascal (Pa); the kPa is also used, but doesn’t need to be memorized.
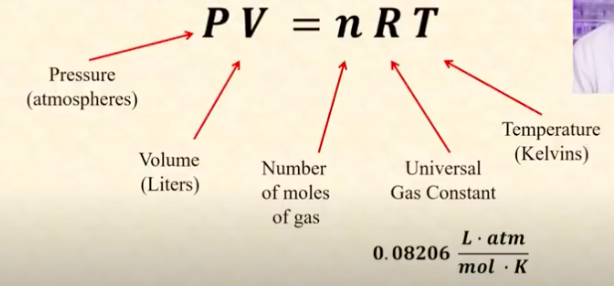
Ideal gas law equation
PV = nRT.
Calculating pressure when gas collected over water: what should we do?
Subtract water vapor pressure from the total pressure.
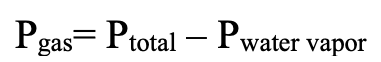
Standard conditions for STP (Standard Temperature & Pressure)
1 atm, 0°C.
Molar gas volume at STP (could also be found using PV = nRT; this is just a handy memorization)
22.4 L/mol
Formula to calculate molar mass of a gas using density
Molar mass = (Density × R × T) / P (molar mass of a gas is DiRTy over P)
Density of a gas @STP
Density@STP = molar mass / 22.4 L/mol
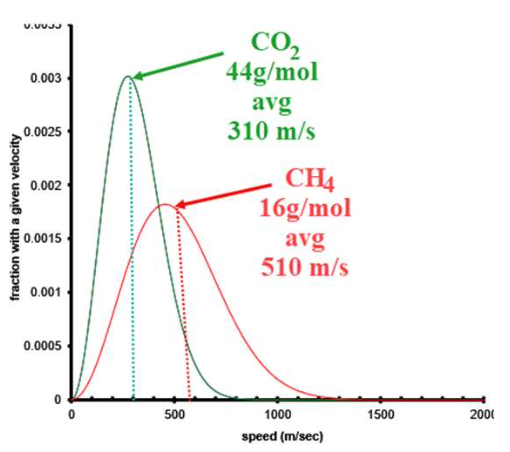
Molecular speed of gases
Lighter molecules move faster.
Average speed on Maxwell-Boltzmann curve
Just to the right of the peak.
Effect of molecular speed on Maxwell-Boltzmann curve
Faster average speed makes the curve wider and more spread out.
Ideal Gas Law Assumptions (5)
negligible volume
high temp
elastic collisions (no energy lost)
constant, random motion
no IMFs
Non-ideal gas conditions
High pressure and low temp.
Non-ideal gas behavior at high pressure
Molecules are closer together, so intermolecular attractions interfere with motion.
Non-ideal gas behavior at low temperatures
Molecules move more slowly, allowing IMFs to dominate.
Source of higher-than-expected pressure in non-ideal gas
Large molecular volume reduces free space for movement.
Lower-than-expected pressure in non-ideal gas
Strong intermolecular attractions cause molecules to stick together.
Partial pressure of a gas in a mixture
Partial Pressure = Total Pressure * Mol Fraction
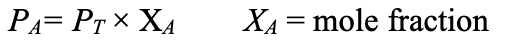
Specific heat capacity of water
4.18 J/g°C (pretty high)
Substances with low specific heat capacities
Metals (typically less than one)
Formula for heat in a calorimeter
q = mass × ΔT × specific heat capacity
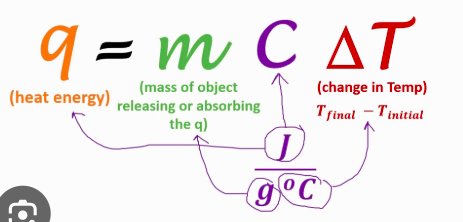
Limiting factor in accuracy of q = mCΔT for sig figs
Usually ΔT because it is the difference between two close temperature values.
Formula for calculating ΔH from calorimetry
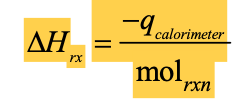
Calorimeter ΔT is positive; what is ΔH and endo/exo?
The reaction is exothermic; ΔH is negative.
Calorimeter ΔT is negative; what is ΔH and endo/exo?
The reaction is endothermic; ΔH is positive.
ΔH definition
The change in potential energy (enthalpy) of a reaction.
Negative ΔH implications
Exothermic; potential energy decreases; bond energy increases.
Positive ΔH implications (endo/exo, potential energy, bond energy, thermodynamic favorability)
Endothermic
potential energy increases
bond energy decreases
reaction is thermodynamically unfavorable unless driven by an increase in entropy (S) or external energy (e.g., electrolytic cell).
Units of ΔH
kJ/molrxn or kJ per mole of product.
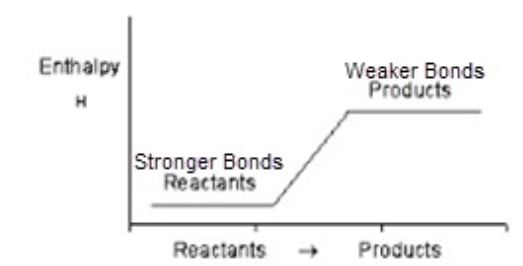
Bonds in an endothermic reaction
Stronger bonds in reactants are broken, weaker bonds in products are formed.
Potential energy in an endothermic reaction
KE is absorbed from surroundings; PE increases.
Exothermic Reaction
A reaction where weaker bonds in the reactants are broken, and stronger bonds in the products are formed (bond energy increases).
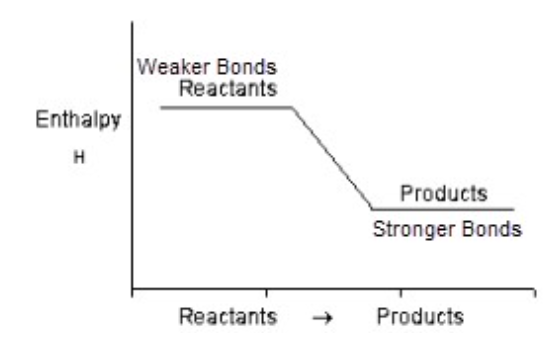
Kinetic Energy in Exothermic Reaction
KE is released to surroundings; the surroundings to get hotter.
Potential Energy in Exothermic Reaction
Potential energy decreases (ΔH = -).
Thermodynamic Favorability of Exothermic Reaction
Exothermic reactions are thermodynamically favorable.
Heat (KE) in Exothermic Reaction
Heat is a product; ΔH is negative.
Example of Thermodynamically Favorable Reaction
A → B + kinetic energy (heat); exothermic
Heat (KE) in Endothermic Reaction
Heat is a reactant; ΔH is positive.
Example of Thermodynamically Unfavorable Reaction (endothermic)
A + kinetic energy → B
What is ΔH(f), Enthalpy of Formation?
The reaction enthalpy to make 1 mole of a substance from its elements in their most common form at 25°C; most are exothermic (ΔH(f) is negative).
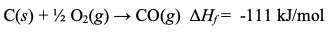
ΔHf of Elements
0 kJ/mol by definition.
Exception to Standard ΔH(f) of Elements
If the element is not in its most common form at 25°C, its ΔH(f) is not zero.
Calculating ΔHrxn Using ΔHf Values
ΔHrxn = ΣΔHf (products) - ΣΔHf (reactants)
Calculating ΔHrxn Using Bond Energies (BE)
ΔH(rxn) = ΣBE (reactants) - ΣBE (products)
the only equation where reactants come first.
Relationship Between Wavelength, Frequency, and Photon Energy
Shorter wavelengths have higher frequencies and greater photon energies.
Unit for Frequency
Hertz (Hz), or 1/second (1/s or s⁻¹).
Calculating Energy of a Photon from Wavelength in nm (2-step method)
Because c is in m/s you must change 100 nm to 100 E-9 m
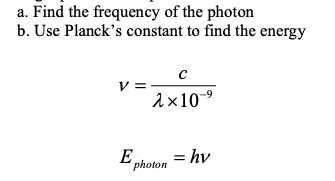
1-step Equation to Calculate Photon Energy from Wavelength in nm
E = h × c / (λ × 10⁻⁹) (Mnemonic: E = hic divided by nine negative lambs)
Converting Photon Energy to Energy per Mole of Photons
E (mol photons) = E (photon) × 6.02 × 10²³
Effect of Absorbed Photons
They transfer energy, causing electrons or molecules to become excited or ionized.
General Effect of Different Photon Energies on Substances
X-rays ionize atoms; ultraviolet and visible light excite electrons; IR and microwaves cause molecular vibrations and rotations.
Visible light: lowest wavelength vs. highest wavelength
400 nm: violet (most energy); 700 nm: red (least energy)
Order of orbital filling up to 5s² (all that’s needed for AP)
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s².
Order of ionization
Grouped by principal energy level: 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² — electrons ionize from the highest energy level down.
Difference between filling and ionization order
Filling order: 4s² filled before 3d¹⁰.
Ionization order: 4s electrons are removed before 3d electrons. Follows grouping order.
Complete electron configuration
Lists all subshells in the order of energy levels (as you would see on a PES graph (3d comes before 4s)).