Acids and Bases & Oxidation Rules
1/31
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
What do acids produce when dissolved?
One dissolved in water acids produced, hydrogen ions
What do bases produce when dissolved?
When dissolved in water basis produce hydroxide ions (OH)
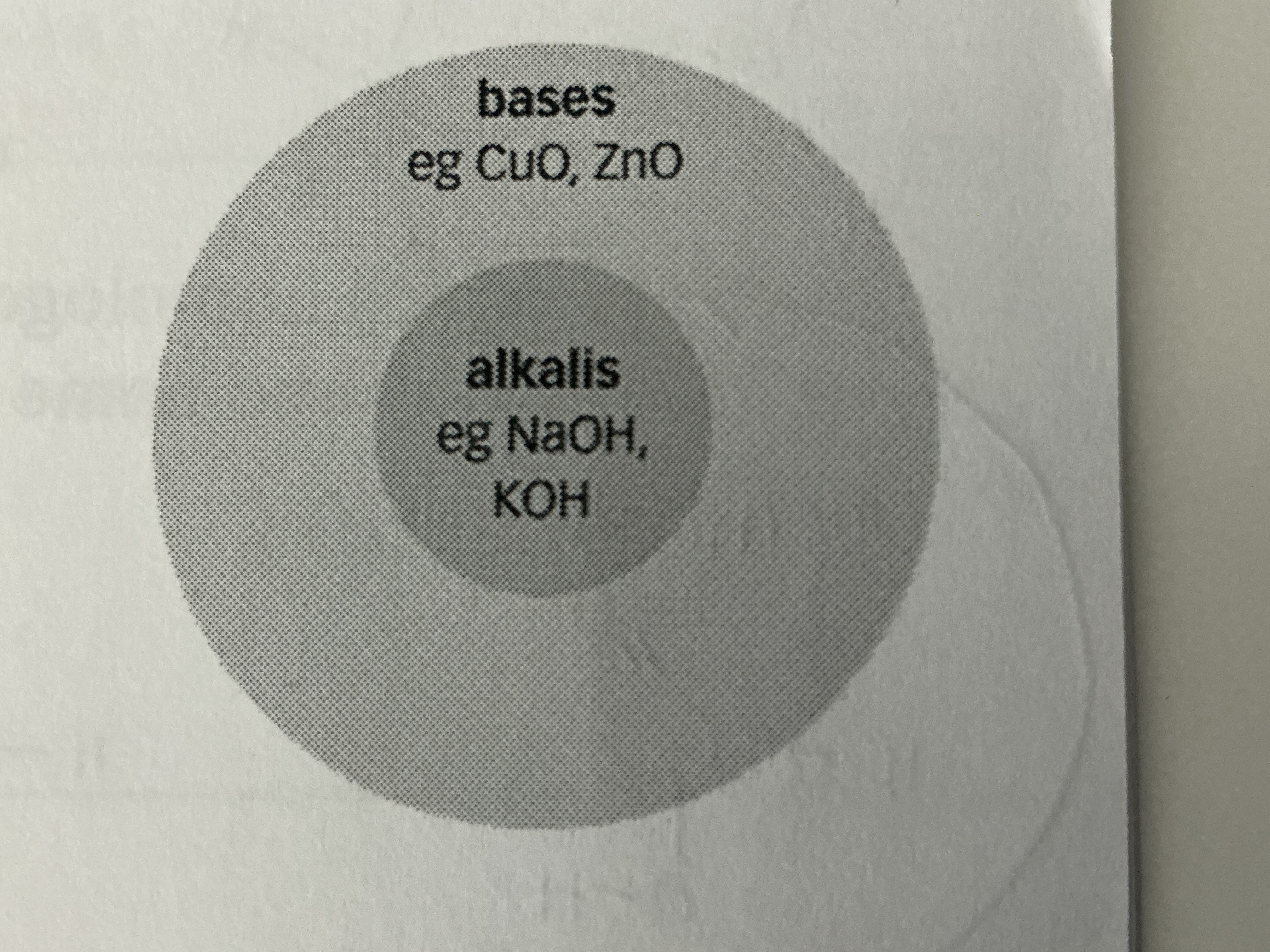
What is another name that a base is called when soluable in water?
If a base is soluble in water, it is called an alkali
Do acids have a high or low pH?
They have a low pH
Do bases have a high or low pH?
They have a high pH
What are the products of a base with an acid?
Neutralization-Salt + water
What are the products of an acid with metal?
Salt + hydrogen gas
What are the products of an acid with carbonate?
Salt + water + carbon dioxide
What happens when MORE solute(s) is dissolved?
The stronger the acid is/the greater than H+ /hydrogen ion concentration
What do bases do with acids?
Bases neutralize acids
What are common examples of strong acids?
HCl, HNO3, H2SO4
What are common examples of weak acids?
CH3COOH
What are common examples of bases?
CaCO3, MgO, CaO
What are common examples of alkali’s (which are all bases but not all bases are alkali’s)?
NaOH, KOH
If an element is pure or uncombined, what is the OX?
0
In a polyatomic ion, the sum of the oxidation numbers of all atoms in the ion must equal what?
The charge on the ion
What is the charge of all alkali metals?
+1
What is the charge of all alkali earth metals?
+2
Other metals/cations generally have oxidation of what?
Oxidation numbers equal to their charge
What is the OX of aluminum?
+3
What is the OX of oxygen generally?
-2
When is oxygen not -2?
In hydrogen peroxide, it is negative one
What is hydrogen’s OX?
+1
When is hydrogens OX not +1?
When it’s bonded to metals, it is -1
What is the OX of fluorine?
-1
What is the OX of chlorine bromine and iodine generally And what’s the exception??
-1, Except it is positive with O or F
What chemical does the cation test use?
NaOH (aq)
What chemical does the ammonium test use?
NH3 (aq)
What chemical does the carbonate test use?
HCl (aq)
What chemical does the sulfate/sulfite test use?
BaCl2 (aq)
What chemical does the halide test use?
AgNO3 (aq)
What is the difference between ammonia and ammonium?
Ammonia- NH3
Ammonium- NH4
The ammonium test for the presence of ammonia (if the damp red litmus paper turns blue there is ammonia gas present)