C20 - 1st Law of Thermodynamics
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
Eint
all energy related to microscopic functions within a system
R constant
R = 8.314 (J)/(mol•K)
R = 8.314 (Pa•m³)/(mol•K)
R= 0.0821 (L•atm)/(mol•K)
Heat
transfer of energy between high temperature and low temperature areas of a system
Calorie
energy required to +1ºC to 1g H2O(l)
1 calorie = 4.186 J
heat capacity
C = Q/∆T
specific heat
c = Q/m∆T
Q = mc∆T
Latent heat
L = Q/∆m
∆m = change in mass of higher-phase substance
Latent heat types
Lvaporization = latent energy for vaporization (liquid → gas)
Lfusion = latent energy for (solid → liquid)
reverse processes are equal in magnitude & opposite in sign (-L)
Work done on a gas (closed environment)
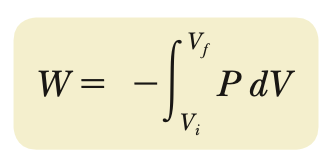
1st Law of Thermodynamics
∆Eint = Q + W
cyclic: Q + W = 0
adiabatic: ∆Eint = W
isovolumetric: ∆Eint = Q
isobaric: W = -P(Vf - Vi)
isotherm (work done)
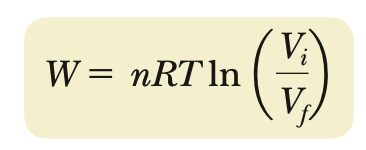
conduction between two faces of block mass
P = kA(∆T/∆x) = kA|dT/dx|
conduction between two ends of a rod
P = kA(∆T/L) = A(∆T/R)
conduction between two ends of a rod (multiple materials)
P = kA(∑(∆Ti/Li)) = A(∆T/∑Ri)
temperature gradient
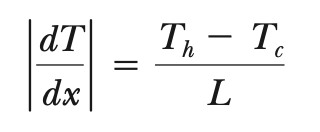
Stefan’s Law
Qradiated = σeAT4
Qnet = σeA(T4 - (T0)4)
σ = 5.7 × 10-8 W/(m2 • K4)
e = emissivity = absorptivity (0 < e < 1)
A = surface area
T0 = ambient temperature
types of transfer mechanisms
conduction (Q)
convection (TMT)
radiation (TER)
Lv (H2O)
2.26 × 106 J/kg
Lf (H2O)
3.33 × 105 J/kg
c (H2O)
4186 J/(kg • ºC)
conservation of energy (isolated heat transfer)
Qcold = -Qhot
Qcold = energy entering the cold substance (+)
Qhot = energy leaving the hot substance (-)
Stefan-Boltzmann constant
σ = 5.7 × 10-8 W/(m2 • K4)
Pa → atm
1 atm = 101325 Pa
m³ → L
1 m³ = 1000 L