Chemistry - Principles of Chemical Equilibrium
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
dynamic equilibrium
the instance in which the forward and backward reactions of a reversible reaction occur simultaneously
Describe liquid-vapor equilibrium
Liquid vapor equilibrium refers to the instance in which a liquid is closed in a container and its surface molecules evaporate, forming a vapour that increases in concentration as evaporation continues to a point that attractive forces between the molecules increase and they begin to condense. Eventually it reaches to a point where the molecules are evaporating as much as they are condensing, forming a liquid-vapor equilibrium.
Describe the characteristics of equilibrium (4)
Dynamic — molecules of reactants are continually being converted to products and products are continually being converted to reactants.
At equilibrium, the rate of the forward and backward reactions are equal.
The concentration of reactants and products at equilibrium do not change and are constant.
Equilibrium only occurs in a close system — one in which none of the reactants or products escape from the reaction mixture, the only exception being reactions in solution where no gases are involved.
Explain the change of the concentration of reactants and products with respect to the direction of the reaction (2)
Going from left to right, products decrease in concentration and reactants increase in concentration
Going from right to left, products increase in concentration and reactants decrease in concentration
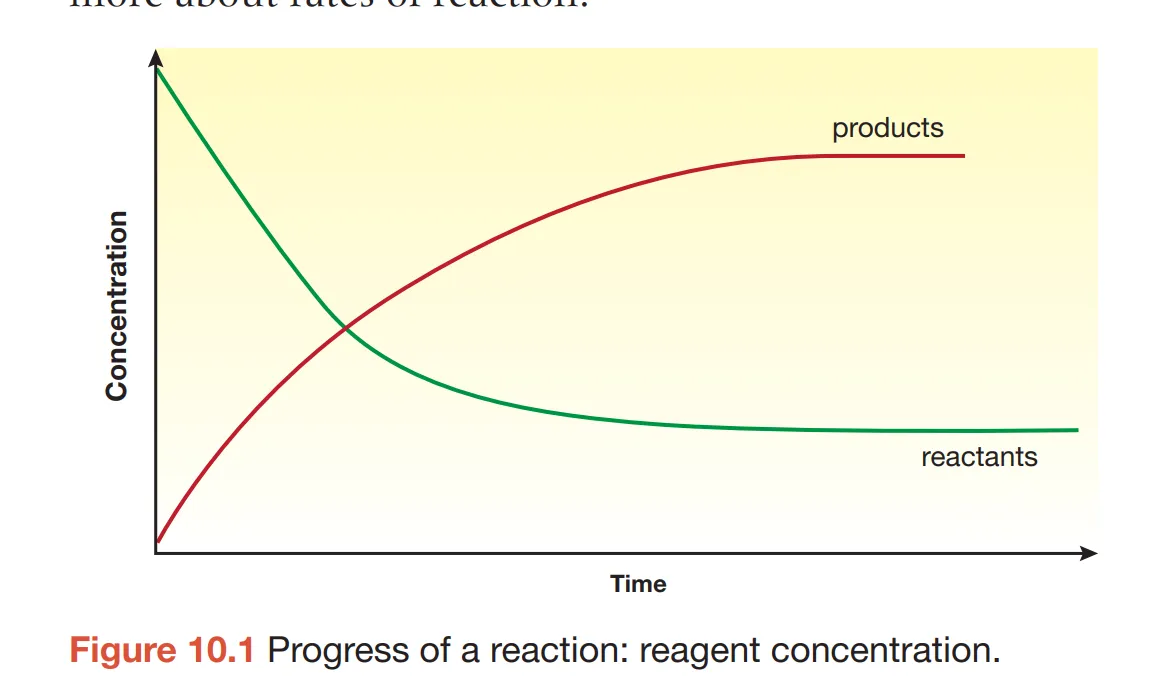
What is this graph representing?
Change in concentration of a product going left to right in a reaction
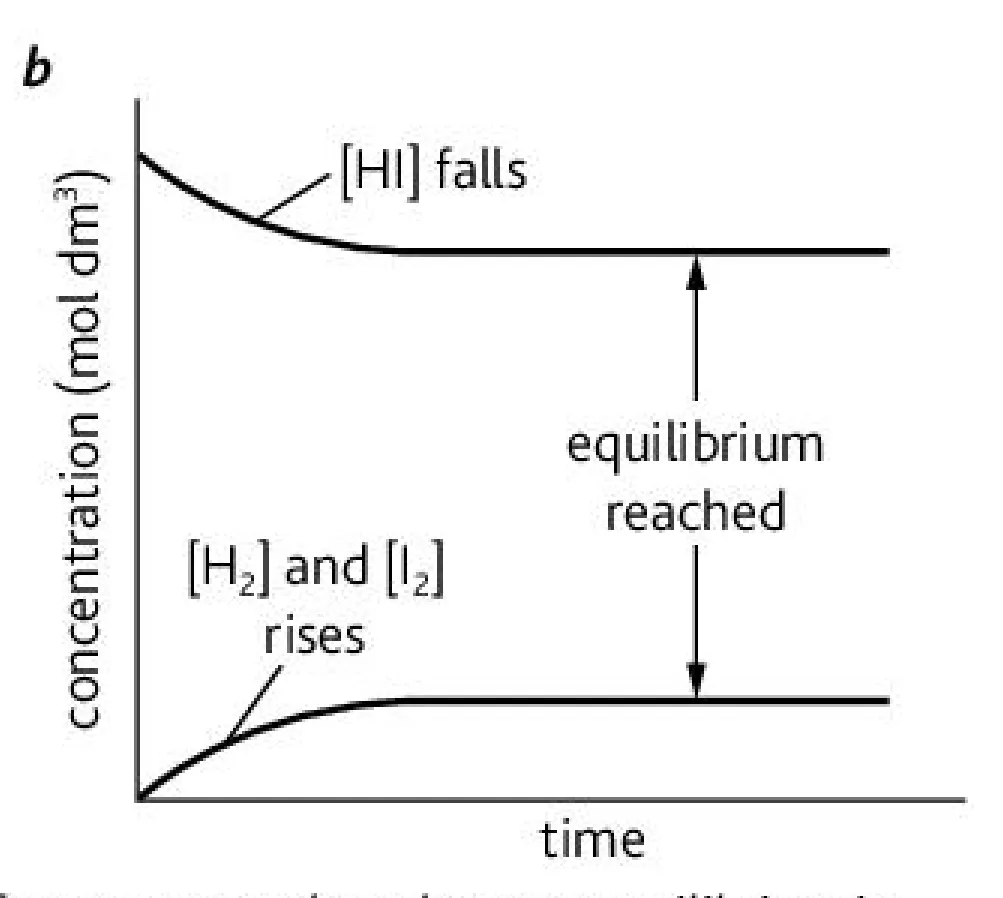
What is this graph representing?
Change in concentration of a product going right to left in a reaction
equilibrium constant (2)
a proportion that expresses the relationship between products and reactants of a reaction at equilibrium with respect to a specific factor
K
Describe Le Chateleir’s Principle and the various factors it affects in regards to equilibrium
Le Chateleir’s Principle states that if one or more factors that affect an equilibrium are changed, the position of equilibrium shifts in the direction which opposes the change. This principle relates to changes in concentration, pressure and temperature.
Describe examples of Le Chateleir’s Principle in respect to concentration (3)
Increasing the concentration of reactant shifts the position of equilibrium to the right and more products are formed.
Increasing the concentration of products shifts the position of equilibrium to the left and more reactants are formed.
Removing products from the reaction shifts the position of equilibrium to the right and more reactants are formed
Describe examples of Le Chateleir’s Principle in respect to pressure (2)
Increasing the pressure, shifts the position to the right where there are fewer gas molecules, opposing the increase in the number of gas molecules per unit volume when the pressure increases.
Decreasing the pressure, shifts the position to the left where the gas molecules are more concentrated, making the molecules move further apart.
Describe examples of Le Chateleir’s Principle in respect to temperature (2)
For an endothermic reaction, increase in temperature increases the value of K꜀ and/or Kₚ so the position of equilibrium shifts to the right and more products are formed
For an exothermic reaction, increase in temperature decreases the value of K꜀ and/or Kₚ, so the position of equilibrium shifts to the left and more reactants are formed
Considering Le Chateleir’s Principle how are the yields of industrial products such as those produced in the Haber and Contact Processes maximized (4)
Pressure increases and more product is formed
Temperature decreases to increase the yield of exothermic processes
Products are removed by condensation to lower concentration so more product is formed
Catalyst is used (but this has no affect on yield, just speeds it up)
K꜀
the proportion between the concentration of the reactants and the products at equilibrium, where c represents the equilibrium constant in terms of concentration
What is the formula for K꜀?
In the reaction, nA + mB ↔ xAB, K꜀ = ([AB]ˣ/[A]ⁿ[B]ᵐ)
How do you solve for K꜀ using the equilibrium concentrations?
Plugging the values into the formula
How do you solve for K꜀ using the initial concentrations and the concentration of a product (at equilibrium)?
Create an ICE table
Define the rate at which the concentration of the reactants and products change at equilibrium by X with coefficients representing the mole ratio
Solve for X using the known value
Use X to find the concentrations at equilibrium
Plug the values into the formula
Kₚ
the proportion between the pressure of gaseous reactants and gaseous products at equilibrium, where p represents the equilibrium constant in terms of partial pressure
How do you calculate partial pressure?
using the formula: mole fraction x total pressure, where the mole fraction = (number of moles of gas)/(total number of moles of gas) and the total pressure (Pₜ) is the sum of the partial pressure of all gases
What is the formula for Kₚ?
In the reaction, nA (g) + mB (g) ↔ xAB, Kₚ = [(pˣAB)/(pⁿA)(pᵐB)]
How do you solve for Kₚ using the molar values at equilibrium?
By plugging the values into the formula
How do you solve for Kₚ using the initial molar values and a known molar value at equilibrium?
Define the change of molar values at equilibrium by X using the mole ratio as a coefficient
Use the known molar value to solve for X
Use X to find the molar values at equilibriums for all substances
Plug the values into the formula