1. Mg, Na reactions with Water
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
Which of sodium and magnesium is more reactive? Why?
Sodium
When sodium reacts, it loses 1 electron to make Na +1, when mg reacts it makes a +2 ion.
It takes less energy to lose one electron than it does to lose two.
How vigorous is sodium reacting with water?
Highly vigorous
List things you can see with sodium reacting with water:
molten ball forming on surface
fizzing, producing H2 gas.
Write the symbol equation for Na reacting with cold water.
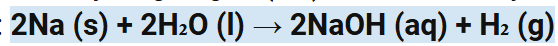
Is the sodium hydroxide produced a strong alkaline? show the pH range.
Yes, strongly alkaline.
pH (12-14)
How vigorous is Mg reacting with water?
Not very vigorous
It reacts very slowly with water
What can you see with Mg reacting with cold water?
Nothing
Is magnesium hydroxide a weak or strong alkaline?
pH range
Weak alkaline solution
pH 9-10
Write the symbol equation for Mg with cold water.
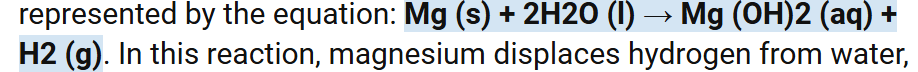
Why is magnesium hydroxide weakly alkaline?
magnesium hydroxide is not very soluble in water, so relatively few hydroxide ions are produced.
Does magnesium react faster or slower with steam?
Faster
Write the symbol equation of Mg with steam.
