Elsaid - pharmacology of antihistamines (copy)
1/34
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
what is a histamine?
an endogenous mediator as a member of the group “Autacoids”
key aspects of histamine:
bioactive amine synthesized from histidine
release to produce local effects (both centrally and peripherally)
role of histamine:
immediate allergic response
regulation of basal acid secretion in the stomach
neurotransmitter and modulator of neurotransmitter release
neurons release histamines as NTs —> block histamine signaling in the brain —> drowsiness
type I immune reaction
IgE-mediated — histamine plays an important role
mechanism:
drug-IgE complex binding to mast cells with release of histamine, inflammatory mediators
mast cells are immune cells with histamines and mediators inside
located in mucous membranes like skin and pulmonary
clinical manifestations:
urticaria (hives), angioedema, bronchospasm, pruritis, vomiting, diarrhea, anaphylaxis
timing of reactions:
minutes to hours after drug exposure
type II immune reaction
cytotoxic
mechanism:
specific IgG or IgM antibodies directed at drug-hapten coated cells in the blood
clinical manifestations:
hemolytic anemia, neutropenia, thrombocytopenia
hemolytic anemia — drug bound to RBC —> antibodies directed against RBC
timing of reactions:
variable
type III immune reaction
immune complex
mechanism:
tissue deposition of drug-antibody complexes with complement activation and inflammation —> tissue damage
clinical manifestations:
serum sickness, fever, rash, arthralgia, lymphadenopathy, urticaria, glomerulonephritis, vasculitis
antibodies in the renal tissue (glomerulus) —> inflammation and destruction of glomerulus
timing of reactions:
1 to 3 weeks after drug exposure
type IV immune reaction
delayed, cell-mediated
mechanism:
MHC presentation of drug molecules to T cells with cytokine and inflammatory mediator release
lot more significant than type 1
clinical manifestations:
allergic contact dermatitis, maculopapular drug rash
timing of reactions:
2 to 7 days after cutaneous drug exposure
histamine: triple response of Lewis
intradermal injection of histamine causes:
red spot: appears within few seconds and maximal at 1 min (direct vasodilator effect of H1 receptor mediated by NO production)
vasodilation of capillaries in the skin —> increased blood flow —> redness
flare or red flush: develops more slowly due to histamine induced stimulation of neuronal reflex causing vasodilation (indirect effect)
stimulation of neural endings —> itching
wheal: swelling at 1-2 min at injection site: histamine effect on blood capillaries increasing permeability
increased permeability —> fluid going into eh tissue —> swelling
mild/cutaneous histamine release symptoms
erythema, urticaria, and/or itching
mild to moderate histamine release symptoms
skin reactions
tachycardia (reflex of vasodilation)
dysrhythmias
moderate hypotension (bc of vasodilation)
mild respiratory distress (histamine causes bronchoconstriction)
severe/anaphylactic histamine release symptoms
severe hypotension (bc of vasodilation)
ventricular fibrillations (heart trying tot pump more blood to make up for vasodilation)
cardiac arrest
bronchospasm (direct bronchoconstriction)
respiratory arrest
histamine receptors
4 subtypes:
H1, H2, H3, and H4
all four receptors are G-protein coupled receptors
activation of H1 receptors causes:
itching, stimulates secretion from nasal mucosa
contraction of bronchial smooth muscles —> respiratory distress
CNS: H1 receptors inhibit appetite and increase wakefulness
H1 and H2: cooperate to induce vascular capillary dilation
H1: increased vascular permeability —> swelling
activation of H2 receptors causes:
gastric acid secretion and H2 receptors may work with H1 receptors in certain types of hypersensitivity reactions
activation of H3 receptors causes:
presynaptic H3 receptors function as auto receptors for histaminergic neurons
autoreceptors = pre-synaptic receptor —> regulates histamine release (stops histamine release once it senses there is enough)
H3 receptor antagonists promote wakefulness

activation of H4 receptors causes:
chemotaxis of immune cells and secretion of pro inflammatory cytokines
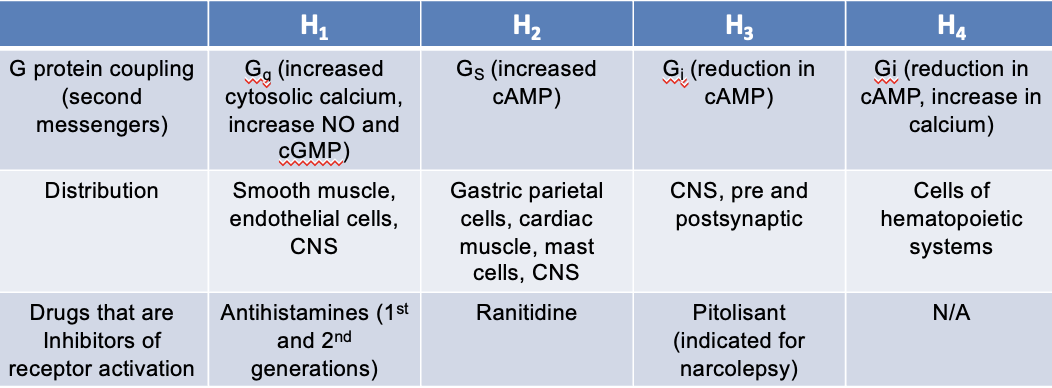
H1 receptors
G protein coupling (second messengers):
Gq — increased systolic Ca2+, increase NO and cGMP
distribution:
smooth muscle
endothelial cells
CNS
drugs that are inhibitors of receptor activation:
antihistamines (1st and 2nd generation)
H2 receptors
G protein coupling (second messengers):
Gs — increased cAMP
distribution:
gastric parietal cells
cardiac muscles
mast cells
CNS
drugs that are inhibitors of receptor activation:
ranitidine — NOT on the market anymore
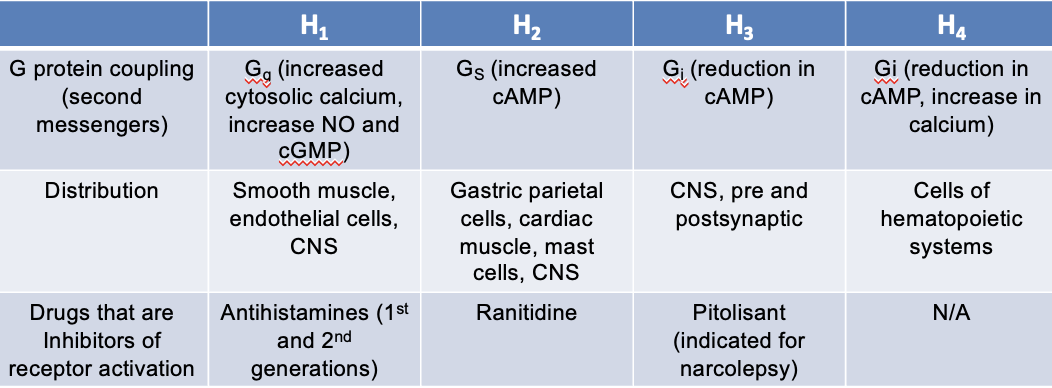
H3 receptors
G protein coupling (second messengers):
Gi — eduction in cAMP
distribution:
CNS
pre and postsynaptic
drugs that are inhibitors of receptor activation:
Pitolisant (indicated for narcolepsy)
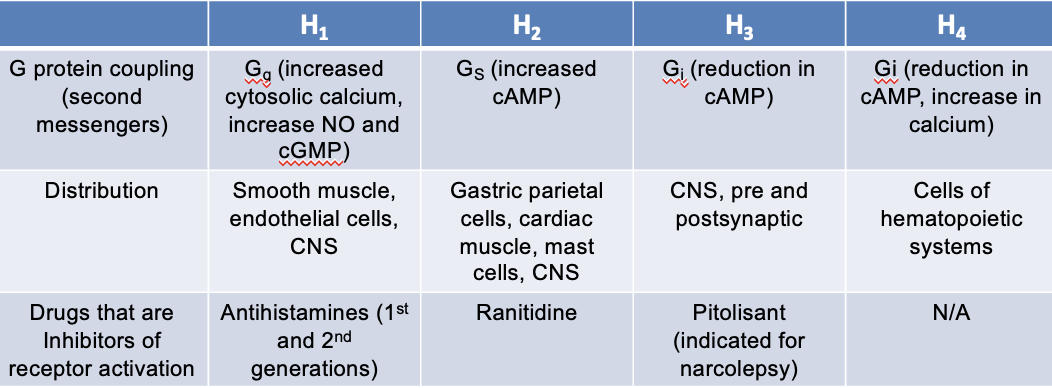
H4 receptors
G protein coupling (second messengers):
Gi — reduction in cAMP, increase in Ca2+
distribution:
cells of hematopoietic systems
drugs that are inhibitors of receptor activation:
N/A
epinephrine
physiologic antagonist —> physiological effect (does NOT act on the same pathway)
antagonizes the effect of H1 mediated bronchial smooth muscle contraction and vasodilation —> DOC for anaphylaxis
𝜶1 receptor agonism: vasoconstriction leading to increases SVR (systemic vascular resistance) and reduction in mucosal edema
β1 receptor agonism: increased inotropy and HR (increases CO)
β2 receptor agonism: bronchodilator and inhibition of further mediator release from mast cells
Norepinephrine does NTO effect bronchial smooth muscle —> NOT DOC
cromolyn sodium and nedocromil
mast cell stabilizers —> histamine antagonism —> reduce histamine release
MOA: prevent mast cell degranulation and release of histamine and other mediators
H1-Antihistamines (H1 inverse agonists)
an inverse agonist —> does NOT compete with histamine for the same receptor
NOT receptor antagonist
histamine (agonist) binds to active receptor and stabilizes the active conformation —> shifts the equilibrium to more active state
antihistamine (inverse agonist) binds to inactive receptor conformation —> shifts the equilibrium to more inactive state (less active state available)
1st vs 2nd generation antihistamines side effects
1st generation:
lipophilic —> goes into CNS —> drowsiness
lots of targets —> more side effects
2nd generation:
acts in PNS and can NOT get into CNS —> less drowsiness
more specific for H1
1st generation antihistamines
Alkylamines:
Chlorpheniramine
Piperazines:
Hydroxyzine, Meclizine, Cyclizine
Piperidines:
Cyproheptadine (has serotonin antagonists properties: used as appetite stimulant and in management of serotonin syndrome)
Ethanolamines:
diphenhydramine, dimenhydrinate, doxylamine
Phenothiazine:
promethazine
Other:
Doxepin (also classified as TCA)
2nd generation antihistamines
Piperazines:
Cetirizine, Levocetirizine
Piperidines:
Loratidien, Desloratidine, Fexofenadine
Other:
Azelastine, Olopatadine (available as nasal spray and eye drops)
pharmacological uses of antihistamines
allergic rhintiis
allergic conjuctivitis
urticaria (hives)
management of cold ysmtpoms
eczema
pruritus (itching) associated with atopic dermatitis
1st generation antihistamines ADEs
CNS H1 receptors:
↓ alertness, cognition, learning, memory, and psychomotor performance
↑ impairment with or without sedation
Muscarinic receptors (cholinergic):
↑ dry mouth
↑ urinary retention
↑ sinus tachycardia
serotonin receptors:
↑ appetite
↑ weight gain
𝜶-adrenergic receptors:
↑ dizziness
↑ postural hypotension
↑ reflex tachycardia
cardiac ion channels (IKr, INa, and others):
↑ QT interval
↑ ventricular arrhythmias
how do anticholinergic drugs increase heart rate?
by increasing SA node firing rate
sympathetic nerves
norepinephrine binds to β receptor and stimulates sympathetic nerves —> increases HR
Ca2+ also going into the cell through the channel ICa
parasympathetic (vagal) nerves
ACh binds to m2 receptor —> decreases HR
K+ also going out of the cell through the channel IKACh
β-blocker effect on HR
β-blockers block β1 receptor —> sympathetic pathway not active and only parasympathetic pathway active —> decrease HR
anticholinergic effect on HR
anticholinergics like Atropine and Benadryl blocks ACh from binding to m2 receptor. —> parasympathetic pathway not active and only sympathetic pathway active —> increase HR
other uses for 1st generation antihistamines
motion sickness: nausea & vomting, dizziness caused by motion
management of acute dystonia associated with central D2 receptor blockade
pathophysiology of antihistamines used for motion sickness
mediated by the inner ear vestibular system) and increased cholinergic/histaminergic neurotransmission
cholinergic and histaminergic neurons (H1 and M receptors) in the inner ear (vestibular system) release ACh and histamines to the cerebellum to the emetic center (medulla) —> causes nausea and vomiting
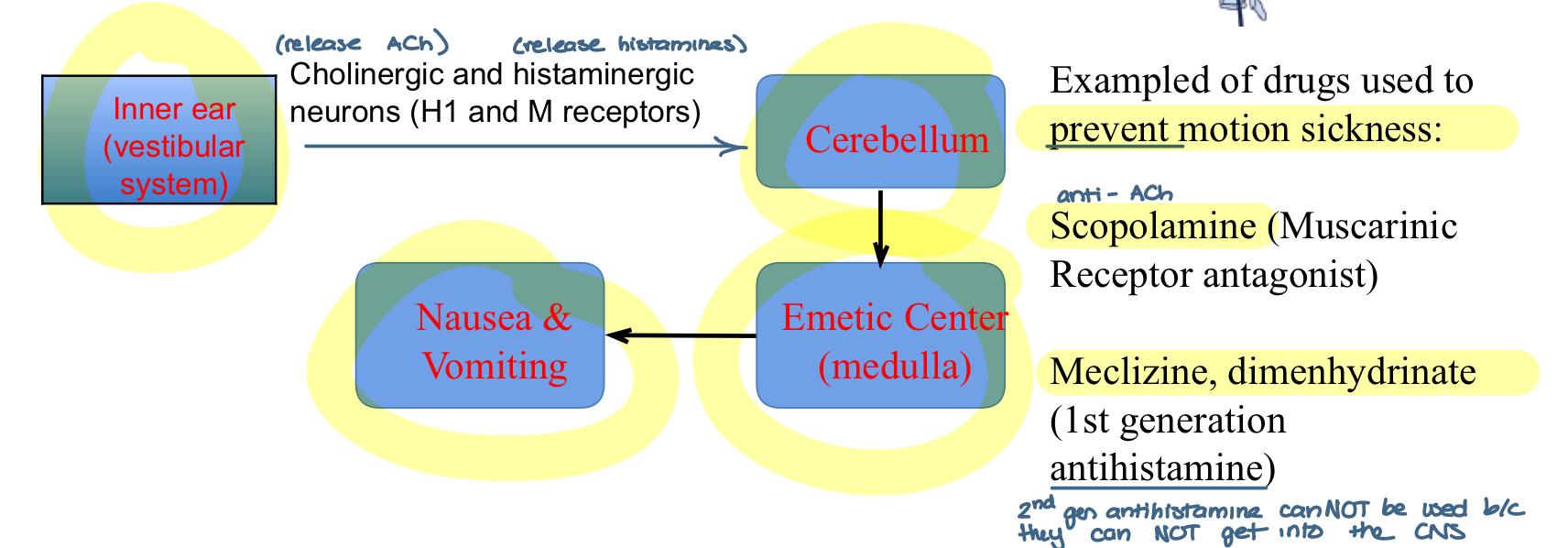
examples of drugs used to prevent motion sickness
Scopolamine (muscarinic receptor antagonist)
meclizine and dimenhydrinate (1st generation antihistamine)
2nd generation can NOT be used bc they cannot get into the CNS
1st generation antihistamines used for management of acute dystonia
management of acute dystonia associated with central D2 receptor blockade
e.g. diphenydramine
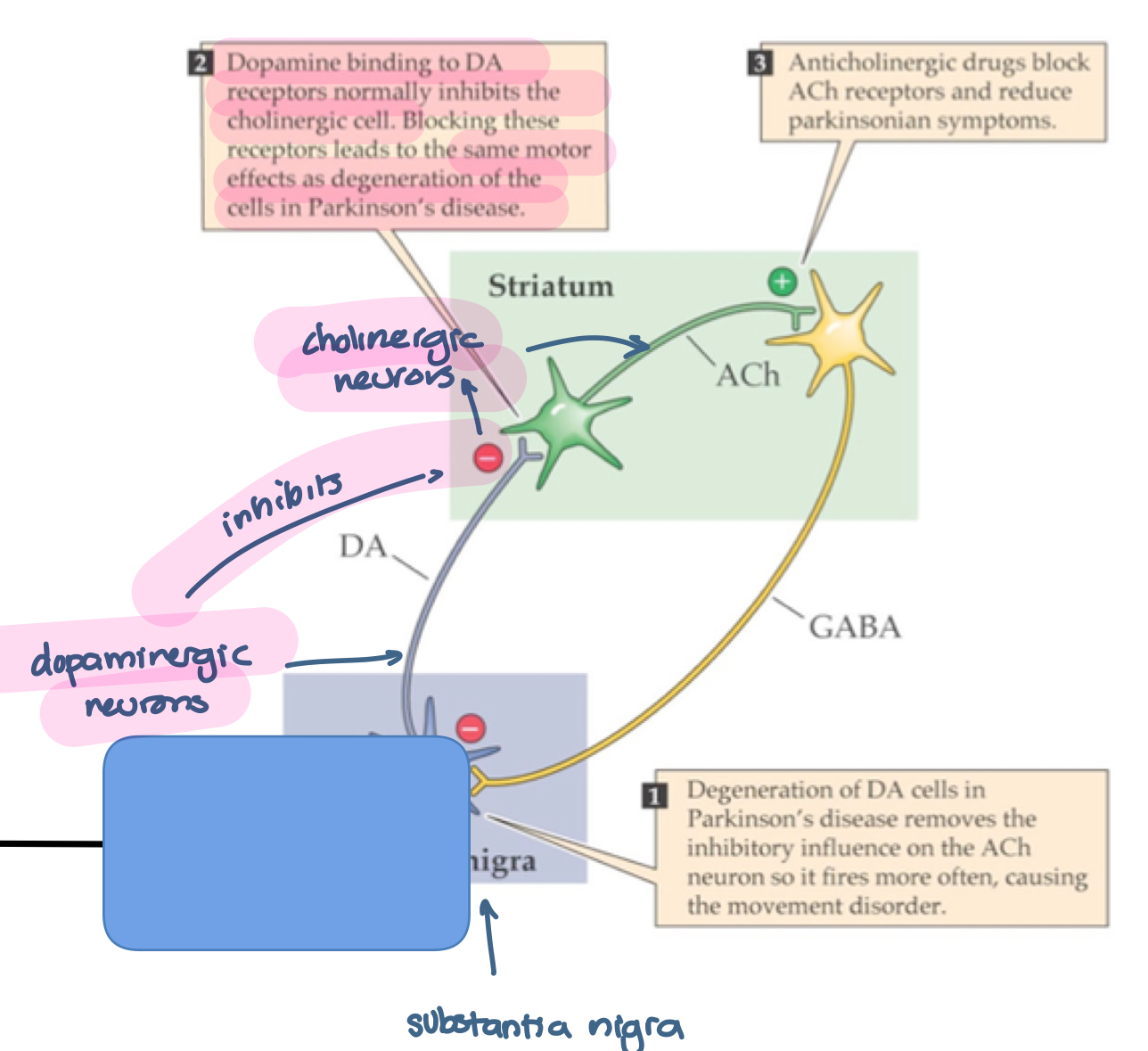
Degeneration of DA cells in Parkinson’s disease removes the inhibitory influence on the ACh neuron so ti fires more often, causing the movement disorder
Dopamine binds to DA receptors normally inhbitors the cholinergic cell. Blocking these receptors lead to the same motor effects as degeneration of the cells in Parkinson’s disease
Anticholinergic drugs block ACh receptors and reduce Parkinsonian symptoms