crude oil
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
25 Terms
what is fractional distillation
process of separating crude oil into parts of liquids based on their boiling points
how does fractional distillation works
crude oil is heated
part of crude oil gets evaporated first and rises to the fractional columns
hydrocarbons condense and are trapped why the fractional columns
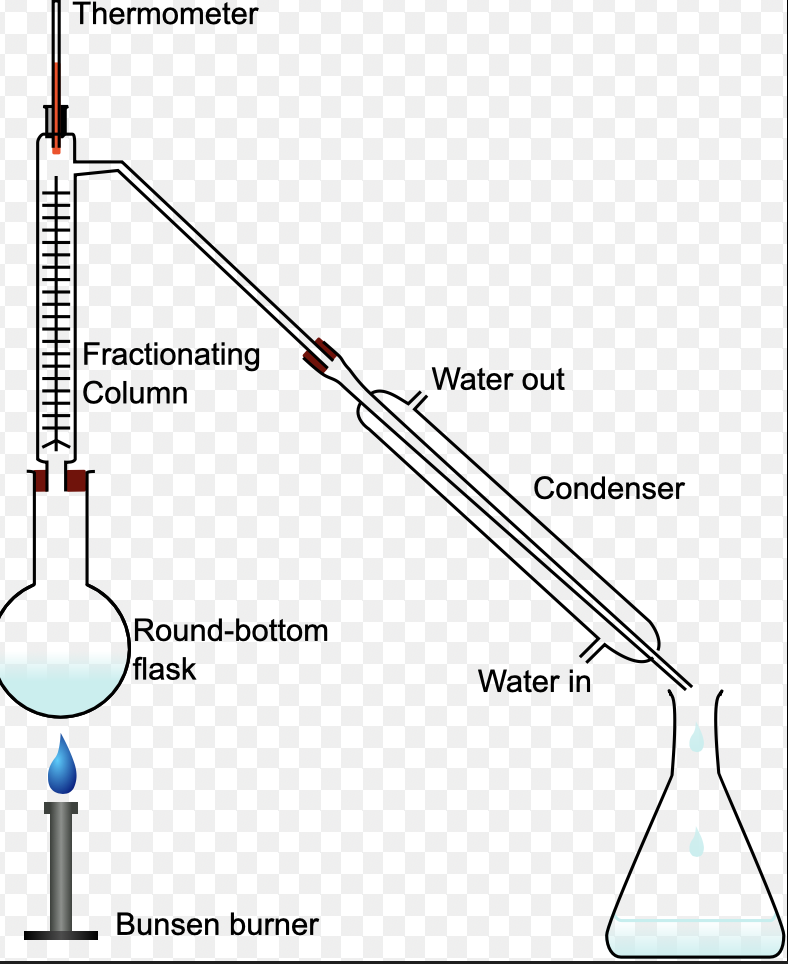
in the part of the fractional column where do low boiling points and high boiling points liquids
low boiling points- high part of the fraction column
high boiling points- bottom part of the fraction column
from bottom to up, what is the order of the crude oil
bitumen
fuel oil
diesel
kerosene
gasoline
refinery glass
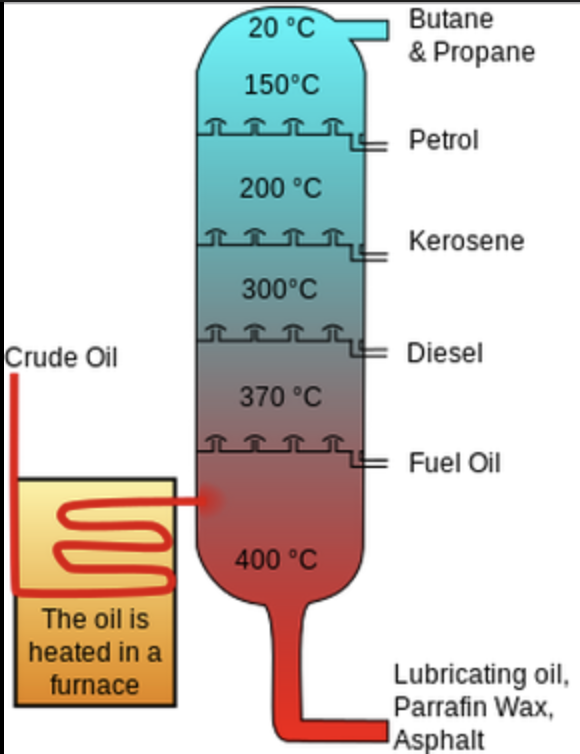
what are the uses for bitumen
road surfacing
waht is fuel oil for
fuel for ships
waht is diesel for
fuel for buses
what is kerosene for
jet fuel
what is petrol for
fuel for cars
what is refinery glass
cooking and heating
what is the trend in crude oils
the longer the carbon chain, the darker the liquid is, higher boiling point, and more thicker.
The shorter the carbon chain is, the liquids colour will have a lighter colour, lower boiling point and less thicker.
define fuel
substance that releases heat energy when burned.
what are the products and reactants in combustion
hydrocarbon + oxygen —> carbon dioxide + water
what is created in incomplete combustion
carbon monoxide
why is carbon monoxide dangerous
becuase it binds onto the haemogoblin in the blood, this decreases the amount of oxygen transferring into the cells that need respiring.
what forms in car engines
since the temperature in the car engines are very high, nitrogen and oxygen inside the car engine can react forming nitrogen oxide
why is nitrogen oxide dangerous
becuase it contributes to acid rain
how does sulfur dioxide form
when buying crude oil that contains sulphur impurities
why is sulfur dioxide dangerous
creates acid rain that
harms aquatic life
damages buildings that are made up of limestone
harms plants
what is catalyst cracking
when the process of cracking is happening but using a catalyst such as alumina at very high temperatures (600-700 degrees)
define alkanes
they are saturated hydrocarbons
define alkenes
they are unsaturated hydrocarbons
how are catalyst used
when the liquid from the crude oil gets evaporated, it goes over the catalyst, this breaks the longer molecules into smaller ones to make them more useful.
what are short alkanes used for
fuel
what are short alkenes used for
making polymers